Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Vaccines
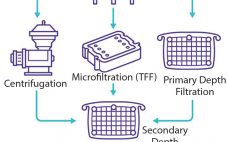
eBook: Viral Vaccine Production — Cultivation of Vero Cells in Packed-Bed Bioreactors
Vero cells are anchorage-dependent cells that are used widely as a platform for viral vaccine production (1). In stirred-tank bioreactors, they are grown ordinarily on microcarriers. Fibra-Cel disks are an alternative attachment matrix because they provide a three-dimensional environment that protects cells from damaging shear forces. However, such disks have not been tested for the cultivation of Vero cells. We tested whether benchtop single-use and glass bioreactors with a packed bed made of Fibra-Cel disks would be suitable for cultivation…
A Vaccine Case Study: Qualifying Redundant Disconnection Technologies As Container-Closure Systems for Long-Term Storage and Shipping
The expanding complexity of biopharmaceutical manufacturing puts increasing pressure on single-use systems to meet the demands of the modern industry’s global footprint. Individual sites within a given organization often are specialized to a fixed number of “modular” process steps (1). Such product segregation increases plant efficiency and output while making the best of staff competencies. But it also can create an additional need for transportation of intermediate or bulk drug substance (BDS) over long distances. Freezing generally is used to…
Going After a Moving Target: New Production Methods Aid in the Flu Fight
The traditional method of manufacturing vaccines for influenza involves infecting hens’ eggs with the virus, then harvesting and purifying the large amounts of virus that they produce as a result. It’s time-consuming and expensive, requiring large specialized facilities for production. With the advent of genetic engineering and decades of improvement in protein production through cell-line engineering and industrial culture, it was only a matter of time before the vaccine industry saw the real value in modern biomanufacturing instead (1, 2).…
Membrane-Based Clarification of Polysaccharide Vaccines
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a Haemophilus influenzae type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (1). Polysaccharides are relatively complex carbohydrates made up of many…
Lot Release and Characterization Testing of Live-Virus–Based Vaccines and Gene Therapy Products
The January 2005 CMC Strategy Forum was devoted to a discussion of live virus vaccines and viral vectors used for gene therapy. The purpose of the meeting was to determine whether consensus positions could be reached among the delegates regarding lot release, stability, characterization, and comparability testing. Part 1 of this two-part report on that meeting describes factors influencing the choices of lot-release assays for vaccines and gene-therapy products (1). Part 2 presents potency testing, characterization, and comparability studies, including…
Culturing a Duck ES-Derived Cell Line in Single-Use Bioreactors: A Rapid, Efficient, and Cost-Effective Vaccine Manufacturing System Based on Suspension Culture
Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies. In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns…
The Importance of the Concentration-Temperature-Viscosity Relationship for the Development of Biologics
JIM DELILLO (WWW.FREEIMAGES.COM) Patient preference and a competitive landscape in the parenteral market have fueled the need for convenient delivery systems and a desire for less‑frequent dosing injections. Monoclonal antibodies (MAbs) often have high dose requirements, so they must be formulated at very high concentrations (1). At low concentrations, an antibody solution’s viscosity increases moderately as a function of protein concentration. But at high concentrations (>100 mg/ mL, depending on the molecule), viscosity increases exponentially (2, 3). Thus, a specification…
Special Report: The Path to Vaccine Profitability
Managing vaccine supply chain improvements involves a complex interaction of laboratories, other facilities, CMOs, and suppliers. Since the business of making vaccines became a commercial proposition, profitability has often been elusive. The economics are difficult: Costs of development and production, already high, are rising. Profit margins historically have been lower than those of other pharmaceutical products, in part because of the complexities of manufacturing and distributing vaccines as well as their stringent safety, testing, and quality requirements.
Bioprocess Advances Drive Vaccine Manufacturing in Developing Countries
Advances in bioprocessing technology hardware and genetic engineering are expanding the geographic options for biologics manufacturing to include developing and emerging economies. Such advances are beginning to permit biopharmaceutical production in regions that previously lacked the technical expertise or quality processes to permit complex operations, monitoring, record-keeping, and oversight. Global demand by countries for in-country production of biological vaccines is increasing, so those products tend to be leading the way in terms of adoption of modern bioprocessing in developing countries.…