Mario Philips is Vice President and General Manager of Single-Use Technologies at Pall Life Sciences. In February, he spoke with BPI publisher Brian Caine and editor in chief Anne Montgomery about Pall’s commitment to enabling continuous processing and its development of single-use technologies. In that discussion, he addressed some major process bottlenecks and Pall’s solution to them, including centrifuge replacements by continuous acoustic wave separation, continuous chromatography with multicolumn chromatography technology platform, and a simplified version of tangential-flow filtration. Read…
Single Use
Flexible Automation for Continuous Unit Operations
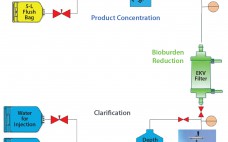
Continuous processing has the potential to provide significant cost and time savings for biopharmaceutical manufacturing, but that potential can be realized only if appropriate automation solutions are available for continuous flow between disparate upstream and downstream operations. Pall Life Sciences’ Allegro MVP system, a fully automated bioprocessing system designed for use in upstream and downstream single-use processing, enables flexible automation and thus facilitates continuous biopharmaceutical manufacturing. This article presents the results obtained using the Allegro MVP system in combination with…
Bridging Polymer Science and Biotechnology Applications with Single-Use Technologies
Implementation of single-use technology in the biotechnology industry is increasing every year. One major interest has been understanding the interaction of extractables with protein and cells for applications ranging from cell banking to biopharmaceutical manufacturing. In October 2015, the Engineering Conference International (ECI) organization hosted a conference in Leesburg, VA, to explore how the science of plastic applies to bioprocessing. The “Single-Use Technologies: Bridging Polymer Science to Biotechnology Applications” meeting brought together experts from different fields to share issues, understanding,…
A Single-Use, Clinical-Scale Filling System: From Design to Delivery
Single-use components have been successfully incorporated into many unit operations for both upstream and downstream processing, from laboratory scale to commercial manufacturing. The development of single-use filling needles has created an opportunity to introduce fully disposable systems into final formulation and filling of drug products (1). One major challenge in replacing a cleanable filling line containing stainless steel needles is to ensure that an alternative system can satisfy all critical performance parameters established for an existing process. In 2012, Merck…
Single-Use Technology Enables Flexible Factories
The biosimilars market is rapidly evolving, with more than 450 biosimilar molecules in development worldwide, and many anticipated transfers of molecules in process around the globe. With US$85 billion of biopharmaceutical products coming off patent by 2020 (1), the driving force to develop biosimilars is strong. The market will be highly saturated, with dozens of biosimilars currently in development for each current blockbuster molecule. We know of 46 trastuzumab biosimilars and 39 rituximab biosimilars in development. Because the biosimilars market…
Outsourcing to Enhance Assurance of Supply: Application of Counterintuitive Supply Chain Strategies — A Case Study
Single-use technologies have transformed biopharmaceutical manufacturing by providing tremendous and proven opportunities to reduce costs, improve flexibility, and shorten cycle times. The expansion of such technologies into commercial production has naturally raised new challenges for both end users and suppliers, thus driving the need for a critical look at risks associated with their use. End users now face a new challenge: how to assess their own supply chains for robust assurance of supply. What is the suppliers’ responsibility in addressing…
Bioprocessing Standards for Single-Use Components Are Moving Forward
With the escalating use of single-use technology in bioprocessing, suppliers have had to rapidly develop disposable components such as fittings, tubing, pumps, sensors, and flexible containers for bioprocessing. Single-use technology is growing so fast that the organizations tasked with guiding its growth are having difficulty keeping up. Contributing to this problem are factors such as company needs, regulatory requirements, market pressures, and costs. That growth has posed considerable concerns for the bioprocessing industry about the presence of organic and inorganic…
The Global Emergence of Single Use
Adoption of single-use manufacturing continues to expand globally and is showing no signs of slowing down. And why should it? The biopharmaceutical industry is challenged to produce safe, effective therapies and vaccines amid the constant pressure to lower cost per dose in addressing healthcare needs of not only the western hemisphere, but emerging markets as well. To meet these challenges, manufacturers are turning to single-use and hybrid systems that incorporate a balance of stainless and single-use equipment. Over the past…
A Single-Use Process for Production of Recombinant Human Follicle-Stimulating Hormone
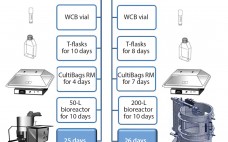
Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein consisting of noncovalently linked α and β subunits. It stimulates the growth of immature follicles in ovaries and primary spermatocytes in testes and thus plays an important role in human reproduction (1). Human menopausal gonadotropin for infertility treatment was first introduced into clinical practice in 1950 (2, 3). Subsequently, treatments with urinary FSH have been replaced by recombinant human FSH (rh-FSH), which has been shown to provide several advantages such as absence of…
Pressure Rating for Bioprocess Single-Use Assemblies
Single-use systems (SUSs) are engineered process equipment solutions for pharmaceutical and biologics production. They offer several key advantages, such as lowering energy costs to reduce utility requirements, minimizing cleaning validation efforts, reducing water and chemical use, and enabling flexibility in manufacturing. Use of SUSs is increasingly popular in almost all fields of bioprocess applications (1, 2). SUSs most commonly comprise components made of polymeric materials, which together create a system or unit operation designed for one-time or campaign use. Single-use…