Biopharmaceutical contract manufacturing organizations (CMOs) were initially enabled when the requirement for a company to file for both an establishment license application and a product licensing application transitioned to the current format of a biologics license application (BLA) submission for biological products (1). The initial focus of such CMOs was to provide large-scale, commercial manufacturing for companies that had already developed and validated bio manufacturing processes. Consequently, CMOs were generally formed as stand-alone service providers that “rented” manufacturing capacity to…
Manufacturing
Best Practices for Technology Transfers Across a Global Network: A Discussion with Patheon’s Paul Jorjorian
A strategic technology transfer plan is the touchstone of global biomanufacturing enterprise, especially for contract service providers that must meet the needs of customers located across several continents. Like their clients, contract development and manufacturing organizations (CDMOs) are facing shortened timelines and cost pressures. They are turning to their process engineers and technology transfer teams to ensure communication with sponsor companies and streamline the transfer of information and critical activities between process development (PD) and manufacturing. In his presentation at…
Outsourcing to Enhance Assurance of Supply: Application of Counterintuitive Supply Chain Strategies — A Case Study
Single-use technologies have transformed biopharmaceutical manufacturing by providing tremendous and proven opportunities to reduce costs, improve flexibility, and shorten cycle times. The expansion of such technologies into commercial production has naturally raised new challenges for both end users and suppliers, thus driving the need for a critical look at risks associated with their use. End users now face a new challenge: how to assess their own supply chains for robust assurance of supply. What is the suppliers’ responsibility in addressing…
Future Manufacturing Strategies for Biosimilars
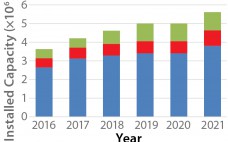
Biosimilars are a relatively new subset of biopharmaceuticals, with the biotechnology industry finally maturing such that off-patent generic-type products increasingly will be entering major markets (1–3). So far, more than 20 biosimilars for a limited number of reference products have been approved in major markets, primarily the European Union. Only two products have been formally approved as biosimilars in the United States. For this rapidly growing industry sector, little consensus or authoritative information is available yet regarding how and where…
Outsourcing Biosimilars Development
A rapid increase in the number of companies working on development and registration of biosimilars has created a significant market for contract testing and manufacturing organizations (CTOs and CMOs) providing outsourced services specific to these products. Biosimilar developers turn to contract organizations when they lack either the internal capability or capacity for conducting certain work as well as when they require additional resources to bring products to market rapidly. A wide range of contract services are available, and each particular…
Critical Factors for Fill–Finish Manufacturing of Biologics
Over recent decades, protein-based therapeutics have emerged as key drivers of growth in the pharmaceutical industry. Drug development pipelines have filled with biologics, and a handful of monoclonal antibody (MAb) products have become some of the best-selling drugs around the world. Production of biotherapeutics is often challenging because of the inherent instability of these large, complex molecules. Their fragile nature has forced manufacturers to change how bulk drug substances (BDSs) are handled and final drug product is formulated, sterile filtered,…
Contract Manufacturing of Cell Therapies: A Conversation with MaSTherCell’s Eric Mathieu and Thibault Jonckheere
The work of developing advanced medical products is spreading around the globe, and with it comes specialized contract services. Far from a “one size fits all” approach to development, and with few platform technologies yet available, contract service providers in the advanced therapeutics space must focus on helping to move promising science into good manufacturing practice (GMP) environments, but with regulatory pathways and eventual harmonization still under development. One company that formed to address the specific needs of cell therapy…
Bioreactor Manufacturing Platforms for Cell Therapies
As an increasing number of cell therapies move into late-phase trials, developers are considering innovative solutions to address scale-up and commercialization challenges. Many of their questions focus on the technologies and engineering strategies that will be needed to optimize their processes, especially bioreactors. At the January 2016 Phacilitate Cell and Gene Therapy World conference, Siddharth Gupta, a scientist at Lonza (Walkersville, MD), talked about the effects of upstream process decisions on product quality in his presentation “Bioreactor Manufacturing Platforms: So…
Special Report on Continuous Bioprocessing: Upstream, Downstream, Ready for Prime Time?
Once an engineering curiosity and smallscale laboratory technique, continuous bioprocessing has evolved in just a few short years to a topic of intense and increasing interest to most bioprocessors. Critics point to a steep learning/adoption curve, but that is nothing new in biomanufacturing.Andrew Zydney is a distinguished professor of chemical engineering at Pennsylvania State University. He has noted these challenges facing continuous processing: commercially unproven unit operations (especially downstream), a lack of equipment robustness, sterility concerns, and uncertain development timelines…
Validation of Controlled Freezing and Thawing Rates: A 16-L–Bag Study
It is well understood that freeze– thaw processes affect the product quality of biopharmaceuticals (1–3). It has been reported that there is no consistent method of controlled freezing and thawing rates for biological formulations (4). Traditionally, ultralow temperature storage chambers that were not designed for freezing have been used to provide an energy state for the environment surrounding the product with very little excess capacity to change the state of the product. This study details a consistent method for controlled-rate…