In our modern biopharmaceutical industry, the maxim “bigger is always better” no longer applies. The industry’s modest virtual model persists in its popularity, emerging as a contemporary method of workplace efficiency. With an absence of manufacturing, virtual biopharmaceutical companies have found themselves unburdened by the multiple layers of bureaucracy that often plague large companies. With such freedom, they have operated much more efficiently in terms of time, resources, product specialization, and finances. As a result, virtual biopharmaceutical companies are growing…
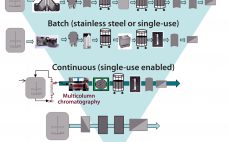
Manufacturing
Outsourcing and Biomanufacturing Challenges for Emerging Therapies: A Roundtable Discussion at BIO 2016’s BPI Theater
The biopharmaceutical industry is increasingly interested in a range of emerging therapies. “We’re really starting to get beyond the monoclonal antibody,” said Patricia Seymour (senior consultant with BioProcess Technology Consultants) in her introduction to a lunchtime BPI Theater roundtable at the 2016 Biotechnology Industry Organization annual convention in San Francisco, CA, this past June. The discussion brought together three industry insiders for strategic outsourcing to talk about emerging biotherapies and their manufacturing challenges: Mark Angelino (senior vice president of pharmaceutical…
Managing Customer and Regulatory Expectations
Partnering with a contract development and manufacturing organization (CDMO) allows drug-product sponsors to turn fixed costs into variable costs. Market forecasting by pharmaceutical companies drives numerous decisions in development programs: sales-force resources, geographic resource distribution, and (of course) manufacturing planning. It is a widely accepted fact in the pharmaceutical industry that accurate forecasting is a challenge, especially for new drug launches. A number of models can be used to develop drug forecasts, but none of these models is perfect. No…
Outsourcing Trends in Biosimilars Development: A Discussion with Niall Dinwoodie (Charles River Laboratories)
No discussion about the future of the biopharmaceutical industry would be complete without assessing the impact of biosimilars. But such discussions no longer focus on whether biosimilars will enter the market, but rather when and how much market share will they take. The rapid progression of biosimilar candidates in company pipelines and the strong biosimilars research conducted by international organizations are strong indications that if your company is not already working within the biosimilars market, it may already be too…
Making the Correct Outsourcing Decisions: BPI Theater Panelists at BIO 2016 Discuss Current and Future Needs for Contract Services
Outsourcing has become a critical part of the industry in general and will play a larger role as companies continue to seek faster and more cost-effective routes to market. On Wednesday 8 June 2016, Gil Roth (president of the Pharma and Biopharma Outsourcing Association, PBOA) chaired a lunchtime roundtable titled “Making the Correct Outsourcing Decisions.” Roth’s association is a nonprofit trade group that represents CMOs and CDMOs through work with Congress and the FDA, with a focus on intra-industry issues.…
Standardized Economic Cost Modeling for Next-Generation MAb Production
Historically, in generating material for clinical testing during antibody process development, emphasis was placed on efficacy, product quality, regulatory compliance, and speed. As the biopharmaceutical industry has matured (and with increasing competition), emphasis has shifted toward cost optimization and manufacturability. Reducing the costs of medicines for patients and payers (thereby broadening access to drugs) is now a key driver during development of new therapies as well as modernizing processes for existing molecules. Cost reduction includes providing robust manufacturing processes that…
Quality By Design for Monoclonal Antibodies, Part 2: Process Design Space and Control Strategies
Process design space and control strategy are two fundamental elements of quality by design (QbD) that must be established as part of biopharmaceutical development and regulatory filings. Like all of QbD, they are interconnected and iterative. Both are based on knowledge gained during product and process development — but both need to be in place (in a potentially very limited form) when a company begins to manufacture drug substance for clinical trials. Part 1 of this discussion appears on pages…
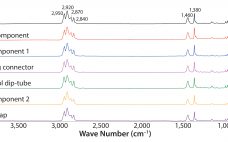
Investigation of Foreign-Particle Contamination: Practical Application of FT-IR, Raman, and SEM-EDS Technologies
The presence of visible foreign particulate matter is considered a critical defect in parenteral products and one of the main reasons they can be recalled (1). Foreign particles present during any stage of manufacturing are considered to be contaminants and can impose a risk to the control of the manufacturing processes (2). For those reasons, particle contamination arising in any manufacturing step initiates a nonconformance or out-of-specification observation. That requires an investigation to identify root cause so as to mitigate…
Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability. The design and manufacture…
Harnessing the Power of Big Data to Improve Drug R&D
Like many other industries, biotechnology is being transformed by the emergence of big data — extremely large data sets that can be analyzed to reveal patterns, trends, and associations — and advanced analytics. Information from multiple sources such as electronic health records, payer claims, and mobile health platforms is growing exponentially. When used and harnessed properly, these data can boost the efficiency of drug research and development (R&D) in three critical areas: early R&D investment, drug development, and personalized medicine.…