One of the fastest growing areas in genome engineering is research using the powerful editing tool of clustered regularly interspaced short palindromic repeats (CRISPR). When paired with the Cas9 (CRISPR-associated protein 9), an RNA-guided DNA endonuclease enzyme from Streptococcus pyogenes, the site-specific prokaryotic immune system can be used to cut and manipulate DNA strands in cells of patients with genetic diseases to treat, or in some cases, prevent such diseases. Within the past couple of years, CRISPR has been shown…
Manufacturing
Cost Analysis of Cell Therapy Manufacture: Autologous Cell Therapies, Part 1
Cell therapies are a growing area of interest for the treatment of a number of indications such as neurological, cardiovascular, and ophthalmological maladies that are refractory to other more conventional drug therapies. A number of cell-based therapy products currently are undergoing clinical trials. The most common target is oncology, which represents 46% of all cell-based therapies through the use of traditional blood-cell and immune-cell–based therapies. For treatment of various cancers, immune cells, lymphocytes (natural killer cells, T cells, and B…
Sourcing Clinical-Grade Human Tissue: Considerations for Supporting Cell Therapy Development and Production
The rapidly developing global cell therapy market poses numerous industry challenges for drug development, process scalability, commercialization, and patient safety. The processes of procuring donated human tissue for clinical applications are fraught with many technical, ethical, and legal issues. Allogeneic cell therapies involving primary cell types such as bone marrow mesenchymal stromal/stem cells (BM-MSCs), hematopoietic stem and progenitor cells (HSPCs), and T and natural killer (NK) cells for immunotherapy applications are especially challenging because of the vigorous process of screening…
Driving Cell Therapy Innovation: Applying Key Lessons from the Evolution and Commercialization of Protein-Based Therapies
After many trials and errors — and milestones — regenerative medicine has become a mainstream part of the biopharmaceutical industry, supported by at least 670 companies and clinics of all sizes. But many experiences in the protein-based industry segment can be leveraged to further improve successful commercialization of advanced therapies. At the 2017 Biotech Week Boston conference, BioProcess International editor in chief Anne Montgomery hosted a panel of industry cell therapy experts to discuss key lessons that can be gleaned…
Cell Expansion with Dissolvable Microcarriers
In recent years we have seen an exponential increase in the number of companies testing and validating new regenerative medicine products. Many of these products are reaching late-phase trials with the potential to receive final approval and marketing authorization from regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). In the past several years, we have seen successful launches of regenerative medicine products, including Holoclar (Holostem Advanced Therapies), Kymriah (tisagenlecleucel, Novartis), Yescarta…
eBook: Viral Vaccine Production — Cultivation of Vero Cells in Packed-Bed Bioreactors
Vero cells are anchorage-dependent cells that are used widely as a platform for viral vaccine production (1). In stirred-tank bioreactors, they are grown ordinarily on microcarriers. Fibra-Cel disks are an alternative attachment matrix because they provide a three-dimensional environment that protects cells from damaging shear forces. However, such disks have not been tested for the cultivation of Vero cells. We tested whether benchtop single-use and glass bioreactors with a packed bed made of Fibra-Cel disks would be suitable for cultivation…
eBook: Alternative Delivery of Biologics — Underdogs Pursue Roads Less Traveled
A number of failures in development of noninjectable delivery methods for therapeutic proteins have caused numerous development programs to crash and burn along with investors’ hopes, dreams, and cash. Most everyone reading BioProcess International is familiar with the issues and challenges: Needles hurt and involve risks to both caregivers and patients. Injections often require administration by trained personnel in specialized settings. But alternative delivery methods are fraught with greater challenges related to dosing, bioavailability (particularly for oral dosing), and inherent…
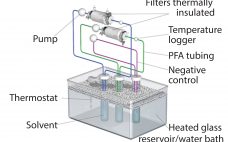
Implementation of the BPOG Extractables Testing Protocols: Comparing USP and BPOG Extractables Data for Autoclaved Polyethersulfone Filters
Benefits of single-use technologies over traditional stainless-steel solutions in biopharmaceutical manufacturing include reductions in set-up times, cleaning/cleaning validation costs, elimination of cross-contamination risks, and smaller operating footprints. But despite increasing adoption of such systems, concerns remain about extractable and leachable (E&L) compounds from plastic single-use systems (SUS) components with the potential to compromise the efficacy and safety of final drug products. Such concerns are magnified by the growing number of SUS suppliers and the complex supply chain for SUS and…
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 2
Monoclonal antibodies (MAbs) are bivalent and monospecific, with two antigen-binding arms that both recognize the same epitope. Bispecific and multispecific antibodies, collectively referred to herein as bispecific antibodies (bsAbs), can have two or more antigen-binding sites, which are capable of recognizing and binding two or more unique epitopes. Based on their structure, bsAbs can be divided into two broad subgroups: IgG-like bsAbs and non–IgG-like bsAbs. We have chosen to focus on the former in this review. Part one provides a…
An Approach to Generating Better Biosimilars: Considerations in Controlling Glycosylation Variability in Protein Therapeutics
The global market for biotherapeutics has expanded extensively over the past decade and is projected to account for more than a quarter of the pharmaceutical market by 2020, with sales exceeding US$290 billion (1). Continued expansion of the biosimilar marketplace has led to many commercial opportunities and technical challenges. The biological systems used to manufacture such drug products are inherently variable — a feature that has important consequences for the reproducibility, safety, and efficacy of the resulting products. Therefore, a…