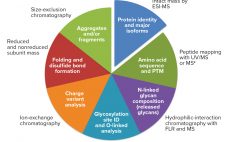
The rapid and cost-effective production of conventional monoclonal antibodies (MAbs) for clinical trials and commercial supply has contributed toward their wide adoption. Production processes have become more efficient because common purification processes are being used across structurally similar MAbs during key steps of process development and manufacturing. Such successful platform approaches can remove unwanted impurities and are stable across processing conditions, irrespective of the MAb being purified. In addition, they are readily available at the required volume to support large-scale…
Manufacturing
CMC Development Platforms and Outsourcing to Reduce Timelines
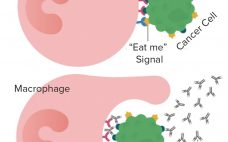
Forty Seven is a company developing novel therapies based on anti-CD47 and other immuno-oncologies. CD47 is called the “don’t eat me” signal that cancer cells give out to escape elimination by the body’s immune system. Qinghai Zhao, vice president of technical development and manufacturing, is one of the scientists working on the company’s magrolimab (5F9) monoclonal antibody that is designed to block the binding of the CD47 signal to the cell receptor SIRP-α while boosting the “eat me” signal that…
eBook: Autologous Cell Therapies: Commercialization Strategies
Autologous cell therapies are derived from a patient’s own stem cells, typically collected from bone marrow. Those cells are then cultured, expanded, and reinfused back into the patient. Unlike allogeneic cell therapies, this process is repeated for each dose and for one patient. The one-to-one process carries several challenges to commercialization, including high development costs, the need to control the risks of manual processing, and compliance with strict timelines. This eBook presents two perspectives on addressing these challenges. The first…
Emerging Treatments for Spinal Cord Damage
Injuries to the spinal cord can cause permanent paralysis and even lead to death, with little or no hope for patients to regain lost function after such trauma has occurred. News of my spinal cord research first came to prominence in the 1980s with a front-page story that chronicles a presentation at the annual Society for Neuroscience meeting (1). That reported the first time that crushed peripheral nerves had been regenerated back into a patient’s spinal cord. Regeneration of spinal…
Biopharmaceutical Growth in China: Bioprocessing Challenges Are Creating CMO Opportunities
In the past decade, the world has seen rapid growth of more than 15% in China’s biopharmaceutical market (1). According to the second edition of BioPlan’s Advances in Biopharmaceutical Technology in China, the most populous country in the world — with the largest patient groups — has a growing economy with its GDP second only to that of the United States. Rapid urbanization and greater access to national healthcare insurance puts China in second place after the United States for…
Control of Protein A Column Loading During Continuous Antibody Production: A Technology Overview of Real-Time Titer Measurement Methods
During production of therapeutic antibodies, harvest titer is measured to monitor product mass loaded onto the protein A capture column. This prevents both column underloading (underusing expensive resin) and overloading (wasting product as flow-through (FT)) while allowing for column yield calculations. Batch production yields a single homogenous harvest pool, thus only one titer measurement (along with volume loaded) is sufficient to determine the mass loaded. During continuous production, however, cell-free harvest (permeate) continuously exits a perfusion reactor and loads a…
Advances and Challenges in Vaccine Development and Manufacture
Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development. Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Although interest in vaccine development and manufacture continues to increase because of the rapid growth of the global vaccine market, this area of the bioprocess industry remains challenging and complex. Here we review the current constraints and complexities in the vaccine industry, specifically related to product development and manufacture. We…
Formulation Development of Microbiome Therapeutics Localized in the Intestines
Scientists have discovered that microorganisms associated with the body play a critical role in human health (1). The intestinal microbiome is the collection of all combined genetic material of microorganisms in the intestines. An intestinal microbiota, a term frequently used interchangeably with microbiome, is the collection of such microorganisms in the intestine. With the volume of research into the human microbiome expanding, so too is the number of examples of its benefits to our health (1). Like diseases of the…
Biosimilarity Assessments: The Totality of Evidence Framework
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1). Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle…
Embedded Particles in Single-Use Bags: Risk to Bag Integrity and Drug Product Purity, or Only a Cosmetic Defect?
When using single-use systems (SUS) to process biopharmaceuticals, preventing drug product contamination from extractables and leachables (E&Ls) and embedded particulate matter (gel particles) in the polymer films used to make bioprocess bags is critical. Using a pressure burst test to assess film integrity, Sartorius Stedim Biotech’s Klaus Wormuth and colleagues compared Flexboy and Flexsafe samples with gel-particle-free materials to assess their potential for contamination. The results showed that only large (2–4 mm2) gel particles affected the burst test results, concluding…