As with many other aspects of biopharmaceutical development, evolving technologies are transforming drug-product development and manufacturing. In a November 2020 report on the Informa Connect “Drug Delivery Partnerships” meeting, BPI senior technical editor Cheryl Scott reviewed how new delivery-device technologies are bringing together companies with disparate expertise to serve patients with chronic conditions better than ever before. The ever-increasing capabilities of electronics and information technology (IT) not only are enabling development of “smart” delivery devices, but also improving the potential…
Manufacturing
Regeneron confident it can scale-up to meet US COVID MAb demands
Regeneron has entered an agreement worth $2.625 billion with the US government and believes it can supply 1.25 million additional doses of its monoclonal antibody cocktail for COVID-19.  The US Department of Health and Human Services (HHS)and the Department of Defense (DOD) will purchase finished doses of the antibody cocktail, REGN-COV2, a combination of casirivimab and imdevimab by June as part of Operation Warp Speed (OWS). The new agreement comes after the Biomedical Advanced Research and Development Authority (BARDA) granted Regeneron…
eBook: Biomarkers — Improving Clinical Studies to Enhance Commercial Success for Biologics
The biopharmaceutical industry continues to invest heavily in technologies for identification of predictive biomarkers. Drug developers want not only to find quantitative evidence that their therapies will work as designed, but also to anticipate which patient populations will respond positively to those regimens. Doing that could streamline clinical trials, accelerate approvals, and ultimately improve patient outcomes. Advances in next-generation sequencing and increases in computational capability now are facilitating biomarker inquiries, especially in the realm of immunooncology. However, predictive biomarkers remain…
Reducing Risk in Bioproduction with Facilities Equivalency
Providing consistency in cell culture media biomanufacturing is critical to supply continuity. Central to this is the development of redundancy and harmonization across a global manufacturing network. These unprecedented times have also highlighted the importance of strategizing for increased and unexpected demand. Read this Special Report to learn about the importance of equivalency and the strategies used to maintain this critical requirement at Gibco cell culture media manufacturing facilities. Fill out the form below to read the complete report and…
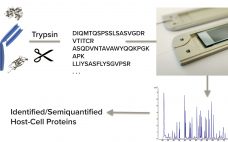
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
Optimization of Processes and Advanced Platforms for Viral Vector Processing
Viral vectors are synonymous with gene therapies, so their development, production, and processing are of upmost importance to all gene therapy researchers and manufacturers. Every year, I look forward to attending the Cell and Gene Bioprocessing and Commercialization conference in Boston and talking to leaders in industry and academia about their current approaches to advancing gene therapies. Like most other meetings this year, the conference was entirely online and had to provide a shortened agenda. Nonetheless, there was no shortage…
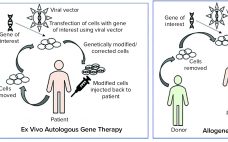
Manufacture and Regulation of Cell, Gene, and Tissue Therapies, Part 1: Chemistry, Manufacturing, and Control Challenges
Cell, gene, and tissue (CGT) therapies and other advanced-therapy medicinal products (ATMPs) have made tremendous progress over the past decade. They are different from other biologics and small molecules because of their inherent complexity and variability. Although many unknowns remain about the development of these products, their clinical success has enabled the CGT therapy and ATMP fields to advance rapidly. We are seeing an increase in the number of marketing authorization applications (MAAs) filed in the European Union and new…
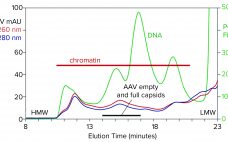
Streamlining Industrial Purification of Adeno-Associated Virus
With its first licensed therapeutic now marketed worldwide (1), adeno-associated virus (AAV) has become a preferred vector for gene therapy. However, unlocking its full potential still poses challenges, many of which are associated with purification. The first involves the transition from upstream to downstream processes. AAV-bearing lysates are laden with debris that foul filtration media and limit or prevent concentration. Another challenge involves reduction of soluble host-cell DNA, which is complicated by its strong association with nucleoproteins. A third involves…
Don’t Be Forced to Accept a Bad Deal During the COVID-19 Crisis
Your boss’s boss just gave you the mandate to procure equipment, components, and raw materials for development and production of a new vaccine. Your goal is to get those as soon as possible — in addition to securing a massive volume for the future. You and your company are in the spotlight, and under significant pressure to deliver. You simply cannot fail. Yet many other players in your industry find themselves in the very same situation. Demand is exploding, and…
Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies, Part 2: Process Modeling and Analytics
Part 1 of this article focused on the first two sessions of a CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies,” which took place on 16–17 July 2018 in Gaithersburg, MD. Those sessions focused on upstream and downstream process technologies and strategies (1). Part 2 highlights the final two conference sessions on process modeling, control, and analytics. Process Modeling and Control The third conference session was “Modeling and Control Strategies.” Advancements in…