As editors, we are fortunate to have the opportunity to listen to biopharmaceutical developers and innovators discuss the intricacies of their work. Typically, we find that the best discussions come from asking two fundamental questions: What need did you observe in the industry that drove your work, and what technologies would help you do your job better? Over the past five years or so, the answers have shifted. Cell and gene therapy (CGT) innovators are focusing on increasingly complex diseases…
Manufacturing
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
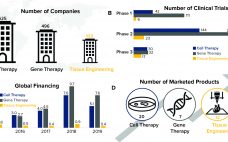
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
The Difficulties of Manufacturing Cell and Gene Therapies At Scale
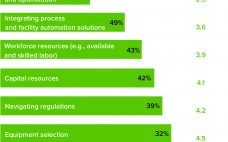
From large-scale manufacturing of one-size-fits-all blockbusters to small-scale processing of personalized therapies, the biopharmaceutical industry has undergone a revolution over the past decade. Among the standout milestones is the development of advanced therapy medicinal products (ATMPs). More than 1,000 of these research-intensive therapies are progressing through clinical trials toward potential commercial manufacturing. Cell and gene therapies (autologous and allogeneic) are targeted for many incurable diseases and conditions, including autoimmune disorders and cancers. Despite the excitement about ATMP potential, developers and…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies: Part 2 — Regulatory Guidances
The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and Japan’s Pharmaceutical and Medical Devices Agency (PMDA) all offer support and guidance for developers of advanced therapy medicinal products (ATMPs). Some agencies have issued guidelines to help companies through different stages of product development — from research and development to marketing authorization and postauthorization activities. Such guidelines are updated regularly as more knowledge becomes available from the development and…
How Capacitance Measurement Can Improve Viral Vector and Virus-Based Vaccine Production
With the increasing development of viral vector and virus-based vaccines, technologies that help to manufacture and scale up these types of vaccine quickly and cost-effectively have become more critical. By accessing this special report from Aditya Bhat, a capacitance technology expert at Aber Instruments, you will find out how in-line capacitance measurement can produce a detailed fingerprint of cell culture processes and how that can benefit vaccine production. From case studies involving baculovirus, AAV, and measles, you will discover why…
Emerging Strategies for Drug-Product Comparability and Process Validation: Part 2 — Validation, Legacy Products, and Lifecycle Management
This two-day CASSS CMC Strategy Forum explored many technical, practical, and regulatory facets of biological drug-product (DP) analytics, process validation, and comparability. Part 1 of this report summarized the discussions on drug-product analytics and comparability in BPI’s March 2021 issue (1). Here we report on day two presentations and discussions on validation, legacy products, and lifecycle management. Session Three: Drug-Product Validation The morning session focused on principles of process validation with examples of challenges specific to drug products. New Risk-Based…
SARS-CoV-2 Hyper-Immunoglobulin: Purification and Characterization from Human Convalescent Plasma
The novel severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) emerged as a major pandemic coronavirus disease in 2019 (COVID-19) and since then has killed many people and paralyzed the global economy (1, 2). With specific antiviral therapeutic agents or antibodies yet to be approved, other antivirals and novel vaccine strategies have been essential to containing the virus and disease transmission. Passive antibody therapy can be used to limit the scope of epidemics by providing patients with antibodies that recognize…
Viral Clearance in a Downstream AAV Process: Case Study Using a Model Virus Panel and a Noninfectious Surrogate
Over the past decade, adenoassociated virus (AAV) vectors have become established as leading gene-delivery vehicles. In 2017, the pipeline for gene therapies included 351 drugs in clinical trials and 316 in preclinical development (1–4). As those candidates advance, significant efforts are being made in process development and manufacturing for viral vectors, with the overall goal of reducing process impurities while maintaining the highest possible process yield. To address that goal, industry suppliers have developed innovative AAV-specific separation technologies. Thermo Fisher…
Rapid Development of Viral Vector Production Processes: Iterative Parameter Optimization
With recent developments and successes in cell and gene therapy, the biopharmaceutical industry is facing increased demand for safe and efficient delivery systems (1). Viral vectors, including adenoviruses (AV), adenoassociated viruses (AAV), and lentiviruses (LV), are among the most common delivery agents because they infect mammalian cells efficiently. Suspension cultures have become a popular choice for robust and scalable viral manufacturing systems. Using stable cell lines that integrate all or part of the viral production elements adds further benefits by…
Ask the Expert: High-Yield mRNA Processing — From Plasmid to Highly Purified Product
Interest in industrial-scale production of messenger RNA (mRNA) has surged amid rapid development of mRNA-based vaccines against SARS-CoV-2. During an 18 February 2021 Ask the Expert presentation, AleÅ¡ Å trancar (chief executive officer of BIA Separations, a Sartorius company) reminded attendees that no platform approach yet exists for mRNA production and that much remains to be learned about manufacturing such products at commercial scales. He described current production challenges and shared BIA’s efforts to devise flexible mRNA purification tools. Å trancar’s Presentation…