Filtration of protein-based biologics is essential for minimizing viral contamination and ensuring product safety and high quality. The tendency of therapeutic monoclonal antibodies (MAbs) and recombinant proteins to aggregate under a number of conditions can complicate selection of a virus filter. An increasing demand for high concentration formulations creates additional challenges. When performing filterability studies and to ensure meaningful virus filter evaluations, downstream process scientists must address factors that can lead to aggregation. This special report on virus filtration by…
Downstream Processing
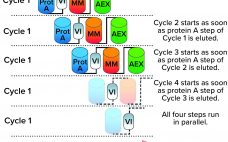
Reducing Downstream Scale-Up Needs: Advances Toward Continuous Downstream Processing
The biopharmaceutical industry generally acknowledges that upstream and downstream aspects of drug-substance manufacturing are experiencing a capacity mismatch. Today, many recombinant proteins can be produced at expression titers of 3 g/L, with some yields exceeding 10 g/L. Such titers represent 100-fold increases in production capability compared with values from twenty years ago (1, 2). Increases in cell-culture density and improvements to perfusion-mode bioreactor systems hold promise for increasing yields further still. Such developments, combined with the broad availability of concentrated…
Mycotoxin Risk Determination: Measuring the Potential for Patient Exposure with Antithrombin Alfa Sourced from Transgenic Goat Milk
Antithrombin alfa is a recombinant human antithrombin developed as an anticoagulant treatment for people with hereditary antithrombin deficiency who are undergoing surgical or childbirth procedures (1). Marketed under the ATryn brand name by LFB SA (Les Ulis, France), antithrombin alfa was approved for use in adults by the US Food and Drug Administration (FDA) in February 2009 (2). Antithrombin alfa is expressed in the milk of transgenic goats and purified through a multistep downstream process encompassing both filtration and chromatography.…
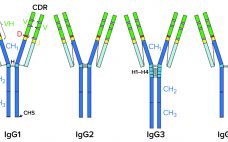
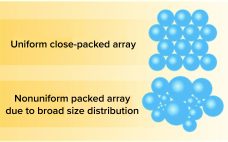
Removing Aggregates and Fragments of Recombinant IgG1: Evaluating a Process Change to Implement Appropriate Chromatographic Media
High–molecular-weight (HMW) and low–molecular-weight (LMW) product variants are critical quality attributes (CQAs) for monoclonal antibodies (MAbs) because they can cause severe immunogenic responses in human recipients. Aggregation is a common problem that can compromise the quality, efficacy, and safety of therapeutic proteins. It can occur at different stages in a biomanufacturing process: during cell-culture–based production, downstream process purification, drug-substance formulation, and storage of bulk drug substances or formulated drug products. Hence, the removal and control of MAb aggregates and fragments…
Improving the Performance of Tried-and-True Chromatography Technology
Efficient and effective downstream processing of biopharmaceuticals reduces manufacturing costs and time. Chromatography is the primary purification method for traditional recombinant proteins, monoclonal antibodies (MAbs), plasmid DNA, and viral vectors. Although interest in membrane separation technologies is growing, traditional resin-based solutions continue to be preferred when high-resolution purification is required. Ion-exchange chromatography (IEC)(e.g., cation and anion-exchange chemistries) and hydrophobic-interaction chromatography (HIC) are established bioseparation technologies. Cation-exchange resins are used widely for MAb polishing and aggregate clearance steps. Anion-exchange (AEX) resins…
AAV Downstream Process and Product Characterization: Integrating Advanced Purification and Analytical Tools into the Workflow
The optimization of the downstream process for Adeno-associated virus (AAV) production with consistent quality depends on the ability to characterize critical quality attributes affecting potency, purity and safety of the final product. As the gene therapy field continues to push products through the clinical pipeline, an increasing need for efficient purification and analytical tools has become evident. In addition, the regulatory space has expanded in parallel to the use of AAV, driving the demand for simple and efficient assays to…
Chromatography in mRNA Production Workflow
Rapid response to global pandemics requires the manufacture of billions of vaccine doses within months. This short timeline must allow for design and testing of active ingredients, development of production and purification processes, clinical evaluations, regulatory filings, and manufacturing. Existing purification methods often have been adopted from laboratory-scale techniques to allow rapid implementation, and those have provided adequate product quality. But future mRNA development will require optimized production and purification processes. Chromatography has been a workhorse of biomanufacturing for decades,…
Purity By Design
Astrea Bioseparations has a well-established modular program to support customer projects from small to large scales with ligands, adsorbents, and chromatography columns that design purity into each process. Demand for increased productivity in biopharmaceutical manufacturing has placed new pressure on downstream purification operations. For recombinant proteins and monoclonal antibodies (MAbs), such pressure stems from significant gains in upstream productivity, particularly from high titers produced using increasingly efficient cell-culture systems. For viral vectors used in gene and gene-modified cell therapies and…
Rethinking Chromatography
Dynamic trends in the biotherapeutic industry are shifting manufacturers towards new modalities and intensified production strategies. This development is supported by ongoing scientific and technical advances in both upstream and downstream processing steps. Downstream processing of new modalities requires chromatography technologies that can handle large, fragile molecules (such as mRNA and viral particles). To maximize speed and productivity, platforms supporting continuous processing will become essential. In this feature, Sartorius discusses current and future concerns for process chromatography operations. They then…
Reducing Cell and Gene Therapy Development Time and Cost with New Purification Strategies
The past 40 years have ushered in the most advanced medicines the world has ever seen, with tremendous improvements in biomanufacturing technologies to enable their development. Advances in production technology have brought significant improvements in upstream productivity, which then caused bottlenecks in downstream processing. Although many bottlenecks have been resolved for most biologics, new modalities such as gene therapies and mRNA vaccines are driving the need for differentiated purification solutions. Meanwhile, pressures to increase efficiency and reduce costs continue to…