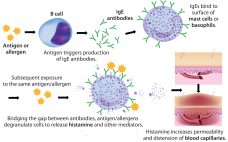
Rapidly increasing use of monoclonal antibodies (MAbs) in the treatment of neoplastic, autoimmune, and inflammatory diseases has led to a dramatic increase in hypersensitivity reactions worldwide, complicating the use of MAbs as first-line therapies and limiting patient survival and quality of life (1). The origins of anaphylaxis are not well understood, though its mechanism is fairly straightforward (Figure 1). It is usually attributed to some undefined intrinsic property or properties of a biotherapeutic — despite the fact that biotherapeutic formulations…
Pre-Clinical and Clinical Trials
The Clinical Side of Biosimilar Development
Biosimilars have become common on pharmacy shelves in Europe. The first biosimilar product — Sandoz’s Omnitrope version of Lilly’s Humatrope (somatropin) — was approved by the European Medicines Agency (EMA) in 2006. In the decade that followed, more than 20 biosimilars have gained regulatory approval in Europe. The first biosimilar monoclonal antibodies (MAbs) — comparators to Janssen’s Remicade (infliximab) — were approved in 2013. The pace of approvals in the United States has been much slower. The US Food and…
Driving Therapeutic Innovations: Academic Institutions Can Help Lessen Development Risks
During the Biotech Week in Boston this past October, I had a chance to talk with David DiGiusto (Stanford University) about his work toward advancing bioprocessing and cell therapy development. I asked him to comment on points from his keynote presentation about how academic research groups can sustainably cycle assets into the biopharmaceutical pipeline. University research departments have long made innovative technologies available for commercial licensing. But in the excerpt below, he details ways in which such groups are further…
Progress Toward Commercial Scale and Efficiency in Cell Therapy Bioprocessing
Regenerative medicine includes both cell and gene therapies. Currently 672 regenerative medicine companies operate around the world, and 20 products have been approved by the US Food and Drug Administration (FDA). Of 631 ongoing clinical trials by the end of 2015 (1), over 40% are in oncology, followed in prominence by cardiovascular and infectious diseases. Here I focus on gene and cell therapy bioprocessing in which the final products delivered to patients are cells. Cell therapies are either autologous (derived…
Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
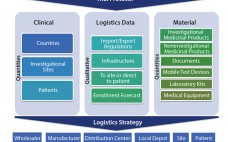
Clinical Supply Chain: A Four-Dimensional Mission
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (1). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers. The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application…
Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 2: Clinical Efficacy, Reimbursement, and Needle-to-Needle Logistics
Cell therapy is an emerging pillar in healthcare with the potential to provide curative solutions to a wide range of indications. The biological complexities through which cell technologies exert their clinical impact (especially those used in immunotherapies for cancer) provide opportunities for novel modes of immune regulation, cell targeting, and payload delivery. Cells also can serve as vehicles for genetic content, which the gene therapy industry is now investigating. Since early 2004, Invetech has worked with organizations dedicated to cell…
Cancer Innovation Forum Calls for Improving US Research “Ecosystem”
Accelerating the commercialization of promising new cancer treatments relies on ensuring that patients — individually and collectively — are actively involved throughout research and drug development. This was the consensus of leading scientists, advocates, and government officials meeting in Washington, DC, at the first national policy forum convened by the Cancer Innovation Coalition (CIC). A collaboration of cancer stakeholder organizations and others working through a national campaign called Project Innovation — which is spearheaded by National Patient Advocate Foundation (NPAF)…
A Critical Mission: Clinical Trial Material Storage and Distribution
As if manufacturing of investigational medicinal products (IMPs) weren’t challenging enough already, the appropriate storage and distribution of sensitive biological products can be an adventurous journey itself — especially if not carefully planned and managed. No one solution fits all situations. Many things must be evaluated during planning stages. For example, what is more important: time to delivery or quality of transport and product integrity until drug can be administered to a patient? It is important to understand key storage…
Biosimilars Awaken CROs
Biosimilars are revolutionizing the bioprocessing industry. Over the years, company CEOs have pushed different models, expanding and closing down huge amounts of internal manufacturing, research and development, and quality control facilities. Sometimes services were pushed to Asia, only to get brought back a few years afterward when a new CEO was appointed. Mergers and acquisitions further complicated those ideas. Consequently, many biopharmaceutical companies now have “gaps” in their drug development service provisions. Filling those gaps will fall on contract research…