The “biopharmaceutical ecosystem” is a multibillion-dollar industry that encompasses large and small drug companies; ancillary providers of services, technologies, equipment, and infrastructure support; and tertiary groups that provide policy direction, regulatory standards, incubator space, and more. This ecosystem is vast and dynamic, evolving constantly as new ideas take hold to push the industry in new directions and present new opportunities for innovation and commercialization. However, many companies operating within this ecosystem struggle to understand their development and commercialization strategies, and…
Intellectual Property
Monetizing Innovations in the Biopharmaceutical Industry
The biopharmaceutical industry has seen strong growth over the past 15 years, with a compounded annual growth rate (CAGR) of 6.7%. However, if you look at that growth in five-year intervals, it becomes apparent that the rates have begun to decline in many countries. Companies have withdrawn somewhat and put initiatives into place to curb further increases in the cost of drugs. The CAGR fell from 8.7% for the period of 2002–2006 to only 3.1% for 2012–2016. In our yearly…
eBook: The Commercial Expression Systems Market — What Has Changed in the Past Decade
A decade ago, BioPlan Associates prepared the findings of its 2008 directory of expression system technologies that were being promoted or considered likely to be suitable for commercial licensing for biopharmaceutical manufacturing (1). Due in part to the relatively slow advances in this critical area of bioprocessing, this study remains perhaps the only directory of biopharmaceutical-relevant expression systems available for licensing. Here I discuss aspects of related bioprocessing technologies that have and have not changed in the past decade. Expression…
Your Brand Is the Patient’s Experience
The future success of biopharmaceutical businesses will depend at least partly on their ability to create meaningful brand experiences from the start of a drug program. By “brand,” I don’t mean logos and taglines. I’m talking about meaningfully unique experiences that directly affect clinical and patient needs — specifically, to address the growing demand for self-administered injectable therapeutics. Whether you are a biosimilar developer trying to carve out differentiated value or a market leader looking at your patent protection in…
Serialization: Background, Justification, Requirements, Timelines, and Readiness Across the Supply Chain
Drug manufacturers are facing unprecedented serialization challenges. Serialization requires weighty consideration and focused strategy for successful commercialization, even for those companies that have yet to bring a product to market. The World Health Organization estimates that 10% of medicines worldwide and up to 50% of drugs consumed in developing nations are counterfeit. In response to increasing drug integrity concerns, more than 40 countries have introduced laws mandating serialization and tracing of pharmaceutical products as they pass through the supply chain.…
Biosimilar Markets and Regulation: Which Countries Are Going All In?
The pipeline of follow-on (biosimilar and biobetter) products in development for the US, EU, and other major markets is very healthy. It includes nearly 800 biosimilars, about three-quarters of which are presumed to be targeted for major markets, and about 500 biobetters in development. Nearly 1,200 follow-on biopharmaceutical products in the development pipeline are intended to compete with more than 100 currently marketed biopharmaceuticals. This is not just an opportunity in the Western world; biosimilars development is expanding globally. But…
Impact of Post-Grant Proceedings on Biologics and Biosimilars
Biologics, primarily therapeutic antibodies and recombinant proteins, represent a growing share of the current drug market, with projected worldwide sales of about US$278 billion by 2020, partly because of their relatively high costs. Indeed, AbbVie’s therapeutic antibody Humira (adalimumab) is currently the top-selling drug in the world by sales but not even in the top 50 by the number of monthly prescriptions. Although the process for marketing generic versions of small molecules under the Hatch–Waxman Act is well understood, the…
India’s Next Steps: Quality Improvements Target International Markets
India’s position as a global participant in small-molecule generic drugs, vaccines, and enzymes has been proven over decades. The country is one of the most populous and fastest-growing regions in the world, both economically and technically. But India’s potential as a biologics participant has not been realized. Its competence as a global biologics producer has not yet caught up. Global industry concerns regarding the country’s position in the (bio) pharmaceutical industry haven’t changed much over the past eight years since…
The Year of Data Integrity: 2015 Brought a Worldwide Focus on Training, System Design and Control, and Data Management
Each year, regulatory agencies from around the world focus on critical aspects of the pharmaceutical quality management system, bringing awareness to the industry and continuing to effect positive change. In the past five years, risk assessments, electronic records, and outsourced activities have been in the spotlight. As 2015 closed out, it was clearly the year of data integrity. In March 2015, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) published its GMP Data Integrity Definitions and Guidance for Industry,…
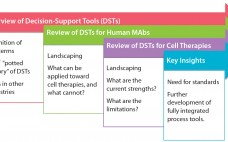
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…