Biosimilars are set to dramatically improve how millions of people access expensive, complex, life-saving treatments by providing equally effective alternatives to innovator products while lessening the cost burden (>30%). Therapeutic Proteins International, LLC (TPI) is a fully integrated manufacturer of biosimilar recombinant protein products that has reinvented conventional approaches to biologic drug manufacturing. Powerful Science Shouldn’t Be Complicated TPI has reinvented the process used to manufacture biological drugs making it simpler by leveraging superior technology and rigorous scientific methods to…
Manufacturing
Who We Are
Major Markets AbbVie Contract Manufacturing serves domestic and global companies seeking to outsource biologics, potent capabilities, drug products, fermentation, prefilled syringe manufacturing, and HME, as well as businesses looking to procure APIs. Outsourcing clients range from mid-size to large pharmaceutical and biotech companies. Services Offered Biologics: AbbVie is a leading biopharmaceutical developer and manufacturer, with a scientific team that offers expertise in process optimization, analytical characterization, and current good manufacturing practice (CGMP) manufacturing to support full development and commercialization of…
Aeras: Leveraging Capabilities to Meet Vaccine Development Challenges
Aeras is a nonprofit biotech focused on the development of TB vaccines. Together with our global research and development partners, we have played an integral role in developing approximately half of all TB vaccine candidates in clinical development around the world. TB is one of the most deadly infectious diseases in the world. It spreads through the air like the common cold. A TB patient with active disease can infect up to 15 people simply by coughing, sneezing, or talking.…
Your Partner from Concept to Commercial
Avid Bioservices is a contract manufacturing organization (CMO) specializing in mammalian cell culture process development and current good manufacturing practice (CGMP) production of clinical- and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to your success, our team is constantly striving to build partnerships that extend well beyond the delivery of product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements. Established CMO with Proven Capabilities nearly two decades of…
Producing Value with Boehringer Ingelheim BioXcellence™
Boehringer Ingelheim BioXcellenceâ„¢ is your dedicated biopharmaceutical contract manufacturer. As a leading biopharmaceutical contract manufacturer with more than 35 years of experience, it has brought 22 biopharmaceutical products to market. Boehringer Ingelheim BioXcellenceâ„¢ offers tailor-made contract development and manufacturing services to the biopharmaceutical industry, providing the entire production technology chain from DNA to fill and finish under one roof at its facilities in Biberach (Germany), Vienna (Austria), Fremont (USA), and Shanghai (China). Boehringer Ingelheim BioXcellenceâ„¢ can secure product supply throughout…
A Profile of Bosch Packaging Technology
Bosch Packaging Technology has been supplying the pharmaceutical industry with final fill solutions for over 30 years. During that time, Bosch has continued to focus on quality and industry-leading customer-focused solutions that provide the best return on investment over time. Filling Systems Bosch is the premier supplier of final fill–finish solutions to the pharmaceutical industry. As a single-source supplier with the industry’s widest portfolio of solutions, Bosch can supply the type and scale of equipment you require. We offer systems…
Expertise, Experience, and Excellence
Expertise Cobra Biologics is a rapidly expanding international contract manufacturing organization (CMO) of biologics and pharmaceuticals for clinical and commercial supply. Cobra has three good manufacturing practice (GMP)-approved facilities, each with expertise tailored to serving our customers across the world. We offer a broad range of integrated and stand-alone contract services, stretching from cell line and process development through to fill and finish for the supply of investigational medicinal products and commercial production. Experience For over 16 years, we have…
Commercial Prefilled Syringe Filling
The commercial prefilled syringe line at Cook Pharmica can process up to 600 syringes per minute, 36,000 syringes per hour, or 70 million syringes per year. The automated, high-speed syringe line is fully enclosed in a barrier isolator to provide the highest level of aseptic processing available by removing the human element from the entire process. With the ability to operate in smaller batches or full-scale campaigning, the prefilled syringe line is capable of bridging clinical programs into commercial production.…
Cell Washing with the LOVO Cell Processing System
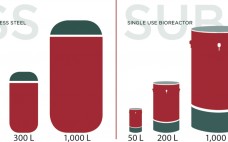
The ability to successfully produce cell-based products is partially dependent on the technologies and instruments available. Volume manipulation, supernatant or culture media exchange, and cell washing of the cell product can present a significant challenge, especially when cell recovery needs to be maximized without compromising viability. LOVO is a multiuse, bench-top instrument that pumps a cell solution through a spinning membrane filtration device to allow for rapid fluid management and fast, efficient cell processing. At its core, LOVO leverages Fresenius…
Focused Process Development for Reliable Biomanufacturing: Experience and Innovation in Cell Culture
Fujifilm Diosynth Biotechnologies (FDB) leverages over 15 years of experience in bioprocess development and manufacturing to successfully deliver robust process designs to its clients. FDB has executed a series of innovation programs to enable rapid development of cell culture processes, including the coming launch of a new Chinese hamster ovary (CHO) expression system. This application note describes how outputs from media screening and upstream process development innovation programs were applied to provide significant productivity and process robustness improvements to an…