Time to market, resource requirements, cost, and flexibility are key considerations in designing purification processes suitable for manufacturing biopharmaceutical products. Over the past decade, many advances have been achieved in disposable processing systems that have allowed for increased processing at a lower cost. That is in part attributable to reductions in necessary resources, changeover costs, and cleaning-validation requirements. Large-scale, prepacked chromatography columns have recently become available for clinical and commercial manufacturing, and they represent a growing trend in the industry.…
Downstream Development
Factors Affecting Sterile Filtration of Sodium-Carboxymethylcellulose–Based Solutions
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (1–3), serves as a suspension aid (4), and stabilizes emulsions (5). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (6, 7). CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree…
Process- and Product-Relate Impurities: Part 1 – Process-Related Impurities An Overview
Introduction by Cheryl Scott The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated…
Special Report: Turning Discoveries into Products — Developability Assessments and Highly Efficient Process Design
High costs and long timelines for biopharmaceutical development are cause for reflecting on how best to allocate resources from the earliest discovery stage through critical go–no-go junctures. With inputs ranging from science, engineering, and economics, the coined term developability becomes the synthesis of answers to such questions as How well does the target represent a disease state? Does manipulating that state bring about improvement? Does the molecule behave as expected in living systems? What can be done about the emergence of independent safety, toxicology, and/or immunogenicity warning signs? Can the molecule…
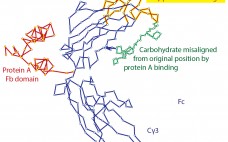
The Secret Life of Protein A
Affinity chromatography with protein A has become the foundation for purification of nearly every therapeutic IgG in commercial production. One of the features most responsible for its success has been its compelling simplicity. IgG binds. Contaminants do not. Load, wash, and elute pure IgG. In the real world, however, protein A does not elute pure IgG. It typically contains several hundred to a few thousand parts per million (ppm) contamination by host-cell proteins (HCPs) and other contaminants. Numerous studies demonstrate…
Identification and Quantification of Heat-Shock Protein 70: A Major Host-Cell Protein Contaminant from HEK Host Cells
Recombinant therapeutic proteins are commonly produced by cell lines such as Chinese hamster ovary (CHO), human embryonic kidney (HEK) 293, murine myeloma (NS0), and Escherichia coli bacterial cells. Host-cell proteins (HCPs) are indigenous proteins produced by those expression hosts and considered to be process-related impurities generated from the cell culture process (1). HCPs are potentially harmful and immunogenic to patients, and they can compromise the stability of protein drugs (2–4). For those reasons, HCPs must be consistently removed or reduced…
Challenges in Implementing Quality By Design: An Industry Perspective
In the fall of 2004, the US Food and Drug Administration (FDA) published a final report entitled Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach (1). This publication set the groundwork for a prospective risk‑based approach to pharmaceutical product development. It was published on the heels of a November 2003 agreement between the FDA and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) to develop an internationally harmonized plan for developing…
Optimization and Scale-Up of HCIC-Based MAb Purification Processes, Part 2
In multistep schemes, hydrophobic charge-induction chromatography (HCIC) has been shown to contribute effectively to clearance of Chinese hamster ovary (CHO) host-cell proteins (CHOPs), DNA, and viruses. When used for capture chromatography, HCIC can provide better aggregate clearance than protein A sorbents can. Chen et al. enhanced clearance of aggregates, CHOPs, and product- related impurities by controlling HCIC based on both pH and the presence of binding-promoting salt in the wash and elution buffers used (1). Taken together with our findings…
An Industrial Platform Solution for Antibody Fragment Purification
Compared with traditional approaches such as chemotherapy and radiotherapy, monoclonal antibodies (MAbs) have become the most successful cancer treatments in the past 20 years (1). With great clinical success in many therapeutic areas, MAbs now account for >40% of the entire biotechnology drug market, and sales are projected to be >US$160 billion over the next few years in the United States alone (2). More than 35 MAbs have been approved for clinical use, and hundreds more are filling industry development…
Optimization and Scale-Up of HCIC-Based MAb Purification Processes, Part 1
Monoclonal antibodies (MAbs) serve important medical needs in cancer treatment as well as that of autoimmune and infectious diseases (1). Antibodies are also widely used in clinical diagnostic assays. They can be coated on solid surfaces to bind specific analytes, conjugated to reporter molecules (either as whole antibodies or fragments) for analyte detection, used in sensitivity panels for lot-release testing, and supplied as positive controls in diagnostic kits (2). Our study evaluates the use of hydrophobic charge-induction chromatography (HCIC) for…