One of the most common posttranslational modifications of eukaryotic proteins is glycosylation. Glycosylation of proteins can affect many biological activities. For therapeutic glycoproteins, it can modify biological activity, targeting, trafficking, serum half life, clearance, and recognition by receptors (1, 2). For such reasons, biomanufacturers must monitor and characterize the glycosylation patterns of their recombinant therapeutic proteins (3, 4). Therapeutic proteins have two main types of glycosylation: N-linked glycans and O-linked glycans (5). Attachment of an N-glycan starts in the endoplasmic…
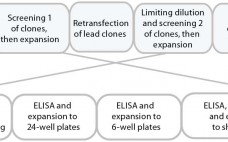
Cell Line Development
Heading for a CHO Revolution: The Need for Cell Line Engineering to Improve Manufacturing Cell Lines
The first recombinant protein licensed for use by the United States Food and Drug Administration (US FDA) was human insulin in 1982 (1). That approval was followed in 1987 by the development of tissue plasminogen activator (tPA), the first complex glycosylated protein generated in mammalian cells to be licensed for therapeutic use. Since then, this area of biology has rapidly expanded in clinics: The FDA approved an average of 15 new biological entities every year between 2006 and 2011 (2).…
Special Report: Turning Discoveries into Products — Developability Assessments and Highly Efficient Process Design
High costs and long timelines for biopharmaceutical development are cause for reflecting on how best to allocate resources from the earliest discovery stage through critical go–no-go junctures. With inputs ranging from science, engineering, and economics, the coined term developability becomes the synthesis of answers to such questions as How well does the target represent a disease state? Does manipulating that state bring about improvement? Does the molecule behave as expected in living systems? What can be done about the emergence of independent safety, toxicology, and/or immunogenicity warning signs? Can the molecule…
Comparison of Concentration Measurement Technologies in Bioprocess Solutions
Biopharmaceutical manufacturing involves complex process steps. Exacting production conditions are typically required to maximize the yield, purity, and quality of biological products. In recent years, process analytical technology (PAT) has been increasingly used to monitor key process and performance parameters in real time. That has enabled better control of production conditions. An important parameter required to achieve consistent results in many bioprocessing steps is solute concentration in process fluids. The Critical Need for Concentration Measurement Many biopharmaceutical manufacturing process steps require measuring…
Rapid Development and Scale-Up of Biosimilar Trastuzumab: A Case Study of Integrated Cell Line and Process Development
Compared with that for new drugs, biosimilar development faces significantly condensed timelines from cell line to first-in-human (FIH) trials. A biosimilar development program needs to accelerate quickly toward preclinical and phase 1 studies; phase 2 studies typically are not required because dose response and other patient-treatment concepts are already established by the original, comparator medicine. Phase 3 studies typically are limited to fewer patients, which ultimately shortens overall timelines and costs. The key challenge remains: demonstrating comparability and high similarity…
Culturing a Duck ES-Derived Cell Line in Single-Use Bioreactors: A Rapid, Efficient, and Cost-Effective Vaccine Manufacturing System Based on Suspension Culture
Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies. In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns…
Essentials in Quality By Design
Quality by design (QbD) is a systematic approach to drug development. It begins with predefined objectives and emphasizes product and process understanding and process control, all based on sound science, data-based decision making, and quality risk management (QRM). As introduced by the US Food and Drug Administration (FDA), QbD brings modern drug development methodologies to chemistry, manufacturing, and control (CMC) teams working on biologics, pharmaceuticals, and vaccines. The innovations associated with QbD are not so much the development concepts (which…
Cell Therapy Bioprocessing Technologies and Indicators of Technological Convergence
The cell therapy industry is undergoing a natural evolution from scientific curiosity into a commercially and clinically attractive opportunity (1). This evolution is by no means complete, and growing evidence suggests that its progression is driving significant developments in cell therapy bioprocessing — notably, convergence. Table 1: 194; () Progressively, bioprocessing technologies primarily used in production of noncell-based products are being evaluated for cell therapy bioprocessing applications (2). Consequently, this process of convergence is leading to an increasing proportion of…
Effective Cryopreservation and Recovery of Human Regulatory T Cells
The list of conditions being targeted by cell therapies is rapidly growing, but commercializing cells for widespread medical use will require standardized laboratory practices. Development processes must be adapted specifically for cell-based drug products. Regulatory T-cell therapy represents a promising new frontier in the immunotherapy of autoimmune disorders, especially for patients who have been refractory to available treatments. Because of intrinsic fragility, cell therapy products can be highly sensitive to variations in manufacturing procedures. Standardization of drug-product cryopreservation and storage…
Assessing Flange Strength and Dimensional Variability
Plastic syringes are viable alternatives to glass syringes in the biopharmaceutical market. They have two main advantages over glass syringes: their break resistance (specifically on the finger flange) and their ability to maintain tighter dimensional tolerances and less variability (because of the flexible molding process). Both attributes are critical when a 1-mL long prefilled syringe is used with an autoinjector device. The high break resistance of plastic syringes can reduce the number of rejected units during a fill–finish process. And…