In a 31 October 2019 “Ask the Expert” presentation, Nicole Wakes (group leader of Abzena’s cell-line development team) observed that drug sponsors often outsource their early upstream activities to a few different contract research organizations (CROs). But that strategy can thwart short timelines and introduce regulatory and financial risks. Wakes described Abzena’s upstream approach, illustrating how partnering with a single, multicompetent CRO from cell line construction through manufacture can streamline workflows. Integrating cell line development and manufacturing in this way…
Cell Line Development
Cell Viability in Bioprocesses: Making a Case for Reevaluation
Trypan blue dye exclusion first was proposed as a means of measuring mammalian cell damages over a century ago in 1917 (1). Despite extensive documentation of its limitations (2), it remains the “gold standard” method of measuring cell viability in common use today. But can this method truly measure viability? And how do we define cell viability, for that matter? Those fundamental questions are linked to whether we refer to cells as “alive” or “dead” in the context of bioprocessing…
Introduction: Reporting from the Frontiers of Cell Line Engineering at BPI Europe and BPI West
Every biomanufacturing process begins with transfection of recombinant genes into pools of cells — followed by a succession of screenings from which will emerge (ideally) a single progenitor cell of the new production cell line. Cast aside will be those cells that do not uptake the correct genetic material, those incapable of thriving in bioprocess conditions, those that fail to produce recombinant protein at relevant levels, and those without demonstrated clonality and relative genetic stability. Over the past several years,…
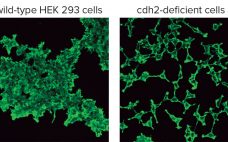
Creating Novel Cell Lines By Genome Editing: Simplifying Cell-Based Assays and Improving Production of Biomolecules
Cultured cell lines have a diverse range of applications. They are used broadly by cell biologists, clinicians, tissue engineers, biotechnology scientists, and bioengineers. The most important uses of cell culture are in the cell-based assays and production of biologically active recombinant proteins. In recent years, genome editing has been used widely to study the structure, function, and localization of endogenous proteins in cultured cells. However, applying the same genome editing techniques to cell lines also could improve the propagation of…
Time Is of the Essence: Optimizing Cell Line Development
Cell line development is a critical upstream step for the manufacture of monoclonal antibodies (MAbs). Cells must be engineered to produce a biologic of interest, and the right clone must be selected to deliver material for preclinical and clinical studies. A high-producing cell line is then needed to support clinical studies and, ultimately, commercialization of the therapeutic. Please fill out the form below to read more. Corresponding authors are Aurore Poles, Global Technical and Compliance Expert, and Guillaume Plane, Global…
A Faster Solution to Hybridoma Screening
Monoclonal antibodies are a rapidly growing class of therapeutics and are used in multiple clinical indications. This highly competitive landscape necessitates the need for rapidly developing next generation antibodies against new challenging targets with improved mechanism of action and pharmacokinetics. Current antibody screening tools such as ELISA, only report on binding to one antigen target at a time; hence these assays are time consuming and require large amounts of target protein. This case study highlights how the Intellicyt® iQue3 system…
Improving CHO Cells for Biomanufacturing
Chinese hamster ovary (CHO) cells have been used in biomanufacturing for decades because of their robust capacity to express a range of proteins, such as therapeutic enzymes and monoclonal antibodies (MAbs) at titers measured in multiple grams per liter of culture. Within the available suite of CHO cell lines, the glutamine synthetase knockout (GS-KO) selection system provides industry-leading speed to the identification of high-producing clones for use in biomanufacturing. The GS-KO selection system allows for identification of multiple-gram/L clones in…
Science Guiding Technology: Cell Line Development and Engineering 2018
Cell line development engineers in the biopharmaceutical industry juggle several, sometimes contradictory priorities. They must present their bioprocessing colleagues with a master cell line that can express a reproducibly high-quality protein product at titers and growth concentrations that will be high enough for manufacturing efficiency — and without those parameters degrading over time. Performing the first step in every bioprocess, these scientists must consider their own budgetary concerns and efficiencies while facing regulatory scrutiny under the 21st-century risk-management paradigm. In…
Myths, Risks, and Best Practices: Production Cell Line Development and Control of Product Consistency During Cell Cultivation
Health authorities are requesting substantial details from sponsors regarding practices used to generate production cell lines for recombinant DNA–(rDNA) derived biopharmaceuticals. Authorities also are asking for information about the clonality of master cell banks (MCBs) and control strategies to minimize genetic heterogeneity. Such requests are prompted by recent reports indicating “nonclonality” for certain production cell lines. To address these and related issues, the CASSS CMC Strategy Forum on “Production Cell Line Development and Control of Product Consistency During Cell Cultivation:…
Rapid Generation of High-Producing Clonal Cell Lines: Using FRET-Based Microfluidic Screening for Analysis, Sorting, Imaging, and Dispensing
Sales of monoclonal antibodies (MAbs) are predicted to be over US$125 billion by 2020 (1). Such revenue potential puts significant pressure on the biopharmaceutical industry to reduce timelines, especially to first-in-human trials. Cell-line development represents a large and critical portion of the early development timeline. Whether a developer is using random or targeted integration for introducing genes into a host-cell genome, the regulatory requirement for addressing monoclonality introduces a time and resource-intensive step in this process. Many different techniques are…