Luina Bio, one of Australia’s most experienced biopharmaceutical contract development and manufacturing organizations, has announced plans to open a new good manufacturing practice (GMP) manufacturing suite. The expansion plans include a new small-scale GMP manufacturing suite that is scheduled to open in the fourth quarter of 2020. The suite will complete the company’s service offerings to customers that require small-scale GMP facilities. “This new suite will allow us to respond to those customers that need a smaller active dose for…
Industry Innovators 2020-2021
Upstream Intensification with High-Density Cell Cryopreservation
In all bioprocess stages, developers and manufacturers aim to intensify as many steps as possible. For upstream processes, the result can be significant productivity gains with an evolution of bioreactor train design that takes advantage of higher titers to compress the train and intensify production outputs. Upstream intensification of production-scale bioreactors has driven bioprocess productivity evolution for several years. Interest has been growing in what can be achieved with laboratory-scale cell expansion, which has remained a fairly static process involving…
Easy Perfusion for Anchorage-Dependent Cell Culture Using an Eppendorf Vessel with Microcarrier Spin Filter
Anchorage-dependent cells such as Vero cells are used widely as a platform for viral vaccine production. Perfusion bioprocesses enable a constant addition of nutrients and removal of byproducts while cells are in a bioreactor. That results in cell densities that are higher than those for conventional batch or fed-batch processes. In the study herein, Eppendorf researchers tested the suitability of a spin filter as a cell-retention device. They cultivated Vero cells on Cytodex 3 microcarriers (10 g/L) in an Eppendorf…
Mitigating Raw-Material Risks During a Pandemic
The COVID-19 crisis has reinforced the importance of having a strong supply chain and a risk-management and business-continuity plan. How can you mitigate your raw material risk, especially during a pandemic? Risks When Choosing a Raw Material Supplier: How do you select your critical raw-material suppliers? What selection criteria and which risks have you identified? To understand risks related to supply, demand, material supplier capacities, and so on, you need information that can come only from communicating with your supplier…
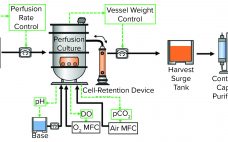
Continuous Biomanufacturing Implementation: Using an Intensified and Integrated Bioprocess Platform
Recent world events have demonstrated now more than ever the growing demand for pharmaceutical biologics that can be made rapidly and in high volumes yet somehow remain affordable. Hence, there is an urgent need to develop a next-generation biomanufacturing solution that provides high-yield, high-quality drug products and is highly flexible and cost-effective. Herein we describe the WuXi Biologics ultrahigh productivity platform (WuXiUP), an intensified perfusion culture process developed to meet the aforementioned need. WuXiUP adopts process-intensification strategies on to traditional…
Simplifying Progress
Never has there been a more critical time to be a company delivering technologies and expertise that helps get new biologics and vaccines to patients faster. Sartorius is that company. Working in partnership with scientists, we help turn research discoveries into real-world medicines. Our bioprocessing equipment and know-how are called on every day to create biologics that treat debilitating diseases, including cancer and arthritis, and make affordable vaccines to prevent common infections. When global organizations such as the Jenner Institute…
Custom Affinity Solutions from Astrea Bioseparations
Astrea Bioseparations Ltd. (Astrea) is the only bioseparations company to offer ligand discovery and adsorbent development services coupled with manufacture and supply of bulk quantities of chromatography adsorbents and production of prepacked chromatography columns to good manufacturing practice (GMP) standards. Our synthetic affinity ligands have been proven over 30 years through numerous examples of successful discovery, development, and delivery of novel affinity adsorbents. Our technology is designed to capture and purify selective target biomolecules — including recombinant proteins, human plasma…
Ensuring Confidence in the Supply of Single-Use Connections
With 25 years’ experience in the biopharmaceutical industry, CPC proudly engineers single-use connection technologies that address biopharmaceutical manufacturers’ requirements for reliability, assurance of sterility, and ease of use. CPC’s innovative line of genderless AseptiQuik sterile connectors allows manufacturers to make quick and easy sterile connections, even in nonclassified environments. All genderless AseptiQuik connectors feature an easy-to-use three-step process that greatly minimizes the risk of operator error and ensures that sterility will be maintained both before and after connection. Fill out…
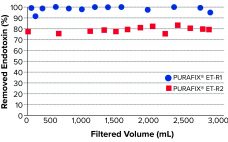
Filtration Solutions for Endotoxin Reduction
PURAFIX ET-R depth-filter sheets are used for efficient and economical reduction of endotoxins from pharmaceutical liquids. Removal of endotoxins is one of the most important and challenging steps in biopharmaceutical processes. Endotoxins — more precisely, lipopolysaccharides (LPS) — are extremely heat and pH stable and therefore withstand sterilization. Purification usually is performed by applying chromatography as a polishing step, which is costly and time consuming. By reducing endotoxin load at an early stage of a clarification process, subsequent purification steps…
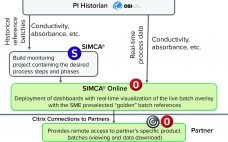
Enabling Digital Chromatogram Review for a Faster and More Reliable Operation
Chromatogram review is a monitoring method used to verify process performance in packed-bed chromatography processes. By observing key process parameters such as chromatography column outlet conductivity or UV absorbance, it is possible to identify the signs of a poorly packed column, resin degradation, or equipment malfunction. Therefore, chromatogram review is implemented as an in-process control (IPC) to decrease variability and identify suboptimal performance, thereby enhancing yield and ensuring high product quality. Fill out the form below to read the…