Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines. Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials,…
2019
Trends in Data Analytics As Organizations Undergo a Digital Transformation
The biopharmaceutical industry is in the midst of an exciting transformation as biologics experience massive growth — even outpacing the small-molecule segment (1). Biologics are predicted to comprise over a quarter of the pharmaceutical market in 2020 (2). At the same time, a plethora of new biologically derived therapy concepts — e.g., cell and gene therapies — are in development. Some biologics classes have become mainstream — e.g., monoclonal antibodies — with biosimilars entering the market and contract manufacturing organizations…
eBook: Viral Vectors for Vaccines — A Virtual Conversation on Production and Analysis
Although today’s vaccines are safer, more effective, and more accessible than they were even 20 years ago, the emergence of new, complex pathogens has exposed limitations in traditional vaccine strategies. Viral vector vaccines (VVVs) hold great promise for confronting those now-intractable pathogens. Combining the best features of live-attenuated and DNA-vaccine approaches, these next-generation prophylactics seek to harness the infectivity of non- or low-immunogenicity viruses to shuttle antigen-encoding DNA from target pathogens into host cells. The resulting transduced cells then initiate…
eBook: Biologics Stability — Lifecycle Management of Drug Products
The biomanufacturing industry’s increasing attention to risk mitigation through quality by design (QbD) and the emergence of complex therapeutic modalities have driven the need for a lifetime-management approach to assuring drug product stability. To that end, industry guidelines have been (or are being) developed to guide the industry toward a “holistic approach” to conducting stability assessments. However, not all methods are stability indicating, and many more industry concerns need to be addressed. This BPI eBook offers perspectives on ICH Q12…
November–December 2019: From the Editor
Over a decade ago, the BioProcess International Conference and Exhibition was created by combining formerly separate IBC conferences on upstream, downstream, analytical, and manufacturing topics for the biopharmaceutical industry. They became tracks in the larger program that reflect the coverage of the magazine after which the event was named. Over time, US West, Asian, and European variations on the theme were added to our yearly calendar. And in the past few years, Biotech Week Boston has evolved by similarly bringing…
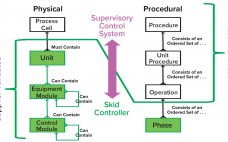
The Value of Plug-and-Play Automation in Single-Use Technology
Automation can improve efficiency, track performance, adjust operations, and liberate operators from mundane routines. Automation requires a flexible set of tools that align well with the inherent flexibility of single-use technology (SUT). Although SUT flexibility enhances a biomanufacturer’s ability to modify operations to meet the needs of today’s dynamic industry, it also increases timelines and costs related to customizing and validating automated additions. We present herein the findings of a team of industry automation experts who are sharing their experiences…
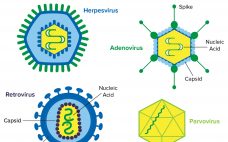
Detection and Clearance of Viruses in the Biopharmaceutical Industry
Viral contamination is a common threat to all animal- and human-derived biopharmaceuticals. This type of contamination can affect any part of a bioproduction process, so biomanufacturers need to perform viral testing studies and incorporate viral clearance methods into their processes. Viral contaminants can come from cell lines (e.g., endogenous retroviruses) or from adventitious (e.g., mycoplasma) introduction during drug manufacturing. Virus testing of master cell banks (MCBs), working cell banks (WCBs), end-of-production cell banks, and bulk unprocessed harvest material is called…
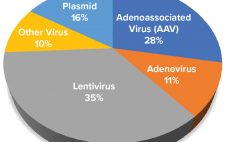
Capacity Analysis for Viral Vector Manufacturing: Is There Enough?
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Analytical Testing Strategies for CAR T-Cell Products
Assay lifecycle development for traditional biopharmaceuticals such as vaccines and monoclonal antibodies (MAbs) has a clearly defined pathway, from preclinical method selection, development, and optimization through the milestones in preclinical phase trials, and finally to postlicensure method evaluations, comparability, and improvements. The analytical development roadmap for nontraditional biologics such as chimeric antigen receptor (CAR) T-cell therapies and gene therapies are not as clearly defined and can present many challenges along the way. Understanding the “what, how, and when” of analytical…
Measure Twice, Treat Once: Navigating the Regulatory Landscape of Assay Development to Ensure High-Quality CGT Products
Cell and gene therapies (CGTs) are a novel and fast-growing class of transformative therapies designed to address gaps in traditional treatment strategies of some of the most severe diseases. By definition, gene therapy “seeks to modify or manipulate expression of a gene to alter the biological properties of living cells for therapeutic use” (1). That can be either an in vivo delivery of a gene or delivery of a gene to a patient’s cells that are manipulated outside of the…