Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines. Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials,…
Industry Innovators 2018-2019
CYLINDRAFlow™ Manifolds: A New Offer That Simplifies Processing Applications
Nordson MEDICAL has launched a new manifold that makes assembly and operation simpler compared with products currently used in the biopharmaceutical industry. This patent-pending product was announced at ACHEMA in Frankfurt, Germany, in 2018. Simplicity is the main benefit of the design and user interface built into CYLINDRAFlow™ manifolds. There is a one-to-one relationship between the inlet port and the five available outlet ports. The large, easy-to-use knob with clearly marked arrows provides a visual indication of which outlet port…
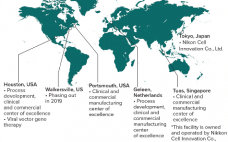
The Next Milestone in Cell and Gene Therapy
The field of cell and gene therapy is transforming the way patients who are diagnosed with cancers or genetic diseases can be treated. Today, the cost of producing these therapies still represents a major hurdle on the way to the commercialization. New technologies are needed that enable robust and cost-efficient manufacturing and yield replicable high-quality medicines. Although the therapeutic opportunities for patients are exciting, the stakes for patients and drug developers are high. We want to be your partner, add…
The Microbial CDMO: Process Development and Manufacturing of Biologics
Wacker Biotech is “The Microbial CMO” — the partner of choice for process development and contract manufacturing of biopharmaceuticals (proteins, vaccines, and live microbial products) using microbial hosts. WACKER’s integrated service portfolio covers molecular biology, process/analytical development, and good manufacturing practice (GMP) manufacturing of biologics for clinical and commercial supply. WACKER operates three state-of-the-art GMP facilities located in Europe (Germany and The Netherlands). Manufacturing lines with two 300-L and two 1,500-L stainless steel fermentation vessels, single-use fermentation lines, matching downstream…
Culture of 3D Cell Aggregates in Perfusion in a DASbox® Mini Bioreactor System
Three-dimensional (3D) culture systems provide cell–cell and cell–extracellular interactions that reproduce the cellular microenvironment in vivo better than typical two-dimensional monolayers. This property is of paramount importance in many applications, including disease modeling, drug toxicity assessment, and manufacturing of stem-cell–based products (1). Cultivation in stirred-tank bioreactors using perfusion mode opens up new possibilities in the cultivation of 3D cell aggregates. Perfusion allows for removal of detrimental metabolites, cell debris, and proteases from the culture as well as the addition of…
Updating Biologics Manufacturing: Using Modern, Single-Use Powder Handling Reduces Time to Market and the Chance of Contamination
Biopharmaceutical companies are striving to produce biologics more efficiently with a lower cost point and fewer employees. Most small-scale media and buffer operations are carried out with small bottles of liquid media. But as processes are scaled up to manufacturing levels, that is no longer a viable option. Costs of shipping liquid are simply prohibitive, so the industry has migrated to using media and buffer in powder form. In fact, 90% of cell-culture media and buffer for sale is now…
Biopharmaceutical Production in Fed-Batch CHO Cell Culture
Chinese hamster ovary (CHO) cells are used widely in the biopharmaceutical industry for the production of recombinant proteins. In times of rapid growth in the biopharmaceutical market with monoclonal antibodies (MAbs) and biosimilars, the use of chemically defined (CD) feeds and culture media for CHO mammalian cell culture is crucial. Currently, strict regulatory norms for the biopharmaceutical industry has led to the wide use of animal-component–free (ACF) culture processes, including CD media and feeds. A business requires significant amounts of…
Complex Protein Production with MaxCyte’s ExPERT Platform for Streamlined Cell Engineering
Complex proteins present a range of production challenges, including low expression levels, degradation, aggregation, a high degree of posttranslational modifications, and the need to use multiple or large expression plasmids. They also can lead to challenges in producing multi-subunit proteins with correct folding and subunit ratios. MaxCyte’s ExPERT platform for cell engineering alleviates those challenges by facilitating the production of complex proteins with high efficiency and cell viability in your cell line of choice. This technical note summarizes four applications…
Enhanced Ergonomics for Large-Volume Fluid Handling: Safe and Easy Operations with Flexsafe® Single-Use Bags
Built on over 30 years of expertise in large-volume fluid processing with 3D single-use bags, Sartorius Stedim Biotech collaborated with many end users for the large-volume Flexsafe® 3D system with enhanced ergonomics, thereby bringing operator safety to the center of successful drug processing. We conducted in-depth interviews to gather and explore operator insights into the usability of large-volume single-use systems. That input helped us improve our product offering with the aim of also improving workplace health and well being. A…
Hydrogen Bond Chromatography – A New Tool for Enhanced Separation of Proteins from Virus Particles and Other Very Large Biologics
CIMmultus ADC monoliths from BIA Separations are the first chromatography products in the field to exploit hydrogen bonding as the primary binding mechanism. These products bring a unique new selectivity to all purification challenges, but they are especially distinctive in their ability to retain large biomolecules more strongly than small ones. They enhance removal of fragments, aggregates, and viruses from proteins, and they enhance removal of proteins and other small contaminants from viruses and other very large biologics. H-Bond ADC…