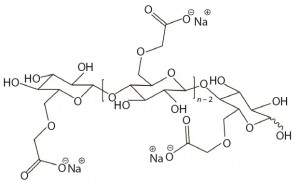
Figure 1: Idealized possible unit structure of sodium carboxymethylcellulose (CMC) with a DS of 1.0; the DS would be 3.0 if all three hydroxyl groups on anhydroglucose unit were substituted. A DS of 3.0 is the theoretical maximum for CMC.
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (1–3), serves as a suspension aid (4), and stabilizes emulsions (5). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (6, 7).
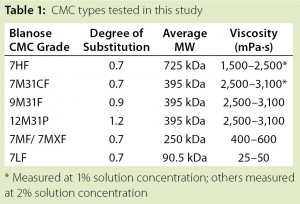
CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree of substitution (DS), which is defined as the average number of hydroxyl groups substituted per anhydroglucose unit (Figure 1). Together, DS and the extent to which carboxymethyl substituents cluster determine functional properties of CMC (e.g., its aqueous solubility). Thus, CMC offers good water solubility above DS 0.6; at a lower DS (e.g., 0.2), CMC retains the fibrous character of its starting material and is insoluble in water (8).
During the manufacture of parenterals, both finished product and precursory process fluids that are labile to gamma irradiation or heat are protected from microbial contamination by filtration. A sterile filtrate typically can be achieved using a 0.2-μm–rated sterilizing-grade filter that has undergone generic validation by its manufacturer with further support by a user’s process-specific validation.
According to their physical– chemical properties, process fluids show varied filterability (filtration behavior in terms of throughput, as a factor of flow rate and filter membrane capacity to blockage). Viscosity enhancers such as CMC can limit the rate of filtration and incur early filter blockage, impacting upon the the practicality and economy of filter use. In some cases, premature filter blockage and increased processing time associated with filtration of CMC-containing solutions has led to concerns over the practicality and economy of using a sterilizing-grade filter for them at all.
Because of those challenges, opportunities for optimizing the filtration of CMC-based solutions are needed. Here we report on a collaboration between Pall Corporation and Ashland Specialty Ingredients to investigate some factors that can affect the filterability of CMC. Ultimately we seek to provide useful data that can help companies engaged in filtration of CMC-based solutions to make informed choices of filters and CMC grade.
Materials and Methods
Tables 1 and 2 summarize the materials used in this study. All types of CMC came from Ashland Specialty Ingredients (Wilmington, DE). Filters from Pall Corporation (Basel, Switzerland) were tested as received. Figure 2 shows the experimental setup.
Preparation of Solutions: CMC particles tend to agglomerate (lump together) when first added to water. For good dissolution, we added CMC to a vortex of vigorously agitated water in preparing test solutions based on the dry mass of CMC (w/w). The rate of addition was slow enough for particles to separate and their surfaces to become individually wetted, but it was fast enough to minimize viscosity buildup of the aqueous phase while the gum was added. Dissolution was complete after an hour of vigorous stirring.
Determination of Viscosity: With test solutions prepared as above, we measured viscosity using a TDVII-LV viscometer from Brookfield Engineering attached by spindle 61 at 60 rpm. Samples were equilibrated at 20 °C before viscosity testing. We calculated the average of three measurements for each solution.
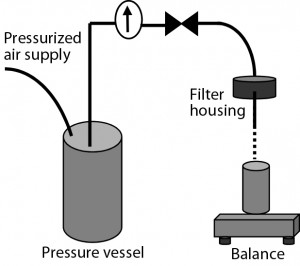
Filterability Testing: Most tests used sterilizing-grade filters without prefiltration. Under those circumstances, 47-mm discs of sterilizing-grade filter membrane were installed in a stainless steel filter housing (Table 2). The housing inlet was connected to an upstream pressure vessel containing a CMC solution to be filtered. The solution passed through the filter by force of air pressure delivered from upstream of the pressure vessel. And filtrate was deposited in a vessel on a balance linked to a system that recorded the change in weight of that collection vessel over time.
When a prefilter was used ahead of a sterilizing-grade filter, the solution to be tested first passed through the prefilter under pressure as above. The resulting filtrate was collected before a second filtration through the final sterilizing-grade filter membrane performed similarly.
All tests were performed at room temperature and at 2-bar constant pressure after ramping up from a starting pressure of 0.5 bar. We terminated each test either when blockage occurred or when sufficient data were collected to indicate that extrapolation of available data would be reliable.
Results and Discussion
Comparison of 0.2-µm–Rated Sterilizing-Grade Filters: We initially subjected Blanose 7M31CF CMC cellulose gum to filterability testing with three different types of 0.2-μm rated, dual-membrane–layer, sterilizing-grade filters differentiated by membrane layer construction and pairing. Table 2 provides information on the construction of each tested filter: Supor EX-grade ECV, Fluorodyne EX-grade EDF, and Supor-grade EBV filter cartridges.
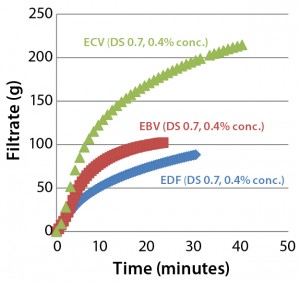
Figure 3: Comparing filtration throughput performance of Supor EX–grade ECV, Fluorodyne EX–grade EDF, and Supor-grade EBV sterilizing filters with Blanose CMC-grade cellulose gum, 7M31CF at 0.4% concentration
Figure 3 shows the filtration performance of each type of filter membranes (47-mm disc format). We found that the Supor EX-grade ECV filter performed better than both the Fluorodyne EX-grade EDF and Supor-grade EBV filters both in total throughput and filtration speed.
Results for the Fluorodyne EX-grade EDF filter and Supor EX-grade ECV filter reveal how two double-layer sterilizing-grade filters (both using an asymmetric PES prefilter layer of the same specification, but with differing downstream membrane layers) can perform quite differently under the same test conditions. Based on its superior performance, we chose the Supor EX-grade ECV filter as our reference membrane for subsequent investigations into the influence of different characteristics of CMC solutions on filterability.
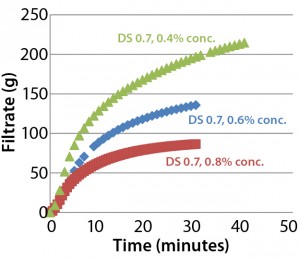
Figure 4: Throughput performance of Supor EX–grade ECV filter with Blanose 7M31CF CMC cellulose gum in water
Effect of Concentration and Viscosity: Viscosity of aqueous CMC solutions increases rapidly with CMC concentration. Figure 4 illustrates the filterability-testing results for different concentrations of Blanose 7M31CF CMC cellulose gum in water through a Supor EX-grade ECV filter. Test-solution viscosities were 29.7 mPa•s (0.4 % w/w), 55.4 mPa•s (0.6 % w/w), and 103.8 mPa•s (0.8 % w/w). Observe the rank order between concentration and filterability. Filterability changes significantly depending on concentration (and thus, viscosity) of a CMC solution: The lower the CMC concentration, the better the filterability.
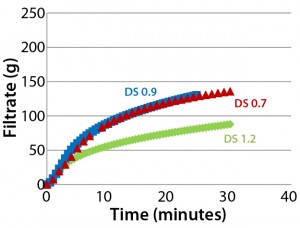
Figure 5: Throughput performance of Supor EX–grade ECV filter using different Blanose CMC solutions with degrees of substitution (DS) in the range of 0.7–1.2; all solutions were adjusted in concentration to have similar viscosity of 40–60 mPa•s.
DS Effect: Figure 5 shows no significant differences between the filterability of DS 0.7 and DS 0.9 solutions, but a solution with a DS of 1.2 showed a higher tendency for early filter clogging. Blanose CMC cellulose gum comes with three different degrees of substitution: 0.7, 0.9, and 1.2. The most widely used types have a DS of 0.7, or an average of 7 carboxymethyl groups per 10 anhydroglucose units. Higher DS grades give improved compatibility with other soluble components such as salts and nonsolvents due to the ease of hydrogen-bond formation between adjacent chains. The higher the degree of substitution, the more rapidly CMC dissolves. However, depending on the DS grade, different stages of solvatization are reached; correspondingly, higher formation of macrogels and microgels occurs with higher DS (10).
Because of the improved solubility from an increase in DS, we expected that Blanose CMC solutions with a higher degree of DS would have less tendency toward filter clogging than lower-DS solutions. But Figure 5 reveals that our hypothesis — that CMC solutions with a DS of 1.2 would filter more easily than their DS 0.7 counterpart — cannot be confirmed. Results indicate reduced throughput with CMC solutions that have a higher degree of substitution. The effect is not related to viscosity because all CMC solutions were adjusted in concentration to have approximately similar viscosity of 40–60 mPa•s. However, we believe that the reduced filterability of the DS 1.2 solution is attributable to the more extensive formation of microgels at higher DS levels and their more pronounced effect on filterability. Our prefiltration study reports (see below) may help to support these findings.
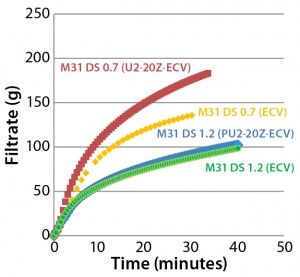
Figure 6: Effects of prefiltration on throughput of Supor EX–grade ECV filter membrane with Blanose CMC cellulose gum, DS 0.7 and DS 1.2
Effects of Prefilter/Filter Construction: Prefilters often are used to improve total throughput and flow performance of sterilizing-grade filters. Figure 6 shows the effect of a 2-μm–rated prefilter on throughput performance of a sterilizing-grade filter for CMC solutions with two different degrees of substitution. We kept viscosities of the two solutions (with higher and lower DS) in the same range by adjusting the CMC concentration accordingly (40–60 mPa•s). For the higher degree of substitution (DS 1.2), prefiltration has almost no effect. However, for the lower degree of substitution (DS 0.7), prefilter use remarkably enhances the sterilizing-grade filter’s performance with respect to volume process and filtration speed.
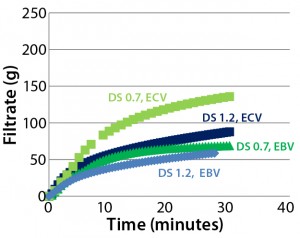
Figure 7: Effects of sterilizing-grade filter membrane construction on filter throughput using Blanose CMC solutions with different degree of substitution (DS)
We also observed improved filterability of lower-substituted CMC when testing the filterability of CMC solutions with differing degrees of substitution through two sterilizing-grade filters: Supor EX ECV and Supor EBV (Figure 7).
The relationship between filterability of a DS 0.7 CMC solution through Supor EBV filters and Supor EX ECV filters is comparable with that of the filterability of a DS 0.7 CMC solution through a single-stage Supor ECV filter and through the same sterilizing-grade filter following prefiltration (Figure 6).
A Supor EX-grade ECV filter consists of an asymmetric polyethersulfone (PES) membrane layer and a symmetric PES membrane layer; a Supor EBV filter consists of two symmetric PES membrane layers with different retention ratings and a coarser upstream membrane. The asymmetric membrane layer in Supor ECV filters enhances filterability performance with lower-substituted CMC solutions (DS 0.7) but has less effect on higher-substituted CMC solution (DS 1.2).
We can explain the behavior observed in both scenarios by considering the difference in the nature of lower-DS CMC solutions compared with those of higher DS, which are assumed to form more microgels. Throughput performance of a sterilizing-grade filter — either with a built-in prefiltration membrane layer or a separate prefilter — appears to be maintained to a greater extent for lower-DS CMC solutions. At the higher DS of 1.2, behavior exhibited by increased microgel formation appears to reduce the effect of prefiltration.
Challenging Assumptions
Filterability of CMC-containing solutions is affected by a number of variables. Our study addresses filtration more specifically in terms of CMC concentration and degree of substitution as well as sterilizing-grade filter membrane structure and the effect of prefiltration. We observed that the CMC property that is likely to have the most significant influence on filtration was degree of substitution. Best throughputs came with 0.7-DS and 0.9-DS CMC grades, whereas CMC with a DS of 1.2 corresponded with significantly reduced filter-throughput performance. That drop in throughput across all types of filters and filter combinations may be attributable to filter clogging caused by increased formation of microgels at the highest degree of substitution. So using CMC with a DS of 0.7 could offer better opportunities for filter optimization than using CMC with a DS of 1.2.
Concerning different filtration types and filter combinations, use of a prefilter can improve performance of a sterilizing-grade filter downstream for DS 0.7 fluids. Process optimization is otherwise achievable with a sterilizing-grade filter that uses an asymmetric membrane layer and has a high flow rate. We found clearly differentiated filtration performance between two double-layer sterilizing-grade filters with the same asymmetric upstream membrane layer but nonmatching downstream membrane layers. Our observation contraindicates any assumption that all sterilizing-grade filters using an asymmetric prefilter membrane will perform comparably. Wherever possible, filterability testing should be performed on more than one grade of sterilizing-grade filter.
References
1 Shah NH, et al. Carboxymethylcellulose: Effect of Degree of Polymerization and Substitution on Tablet Disintegration and Dissolution. J. Pharmaceut. Sci. 70(6) 1981: 611–613.
2 Wahab A, et al. Formulation and Evaluation of Controlled Release Matrices of Ketoprofen and Influence of Different Co-Excipients on the Release Mechanism. Die Pharmazie 66(9) 2011: 677–683.
3 Khan KA, Rhodes CT. Evaluation of Different Viscosity Grades of Sodium Carboxymethylcellulose As Tablet Disintegrants. Pharm. Acta. Helvetica 50(4) 1975: 99–102.
4 Hussain MA, et al. Injectable Suspensions for Prolonged Release Nalbuphine. Drug Dev. Indust. Pharm. 17(1) 1991: 67–76.
5 Adeyeye MC, et al. Viscoelastic Evaluation of Topical Creams Containing Microcrystalline Cellulose/Sodium Carboxymethyl Cellulose As Stabilizer. AAPS PharmSciTech. 3(2) 2002: E8.
6 Ernsting MJ, et al. Synthetic Modification of Carboxymethylcellulose and Use Thereof to Prepare a Nanoparticle Forming Conjugate of Docetaxel for Enhanced Cytotoxicity against Cancer Cells. Bioconjugate Chem. 22(12) 2011: 2474–2486; doi:10.1021/bc200284b.
7 Sun, et al. Cytokine Binding By Polysaccharide–Antibody Conjugates. Mol. Pharmacol. 7(5) 2010: 1769-77; doi:10.1021/mp100150z.
8 Borsa J, Racz I. Carboxymethylcellulose of Fibrous Character, a Survey. Cellul. Chem. Technol. 29(6) 1995: 657–663.
9 Niu CH, Chiu YY. FDA Perspective on Peptide Formulation and Stability Issues. J. Pharmaceut. Sci. 87(11) 1998: 1331–1334.
10 Gruber E. Mikrogelpartikel in Lösungen von Cellulose und Cellulosederivaten. Cellul. Chem. Technol. 13(3) 1979: 259–278.
Barbara Frei-Rutishauser, PhD, is a scientist at Pall Corporation in Basel, Switzerland. Christian Muehlenfeld, PhD, is a pharmaceutical scientist, and Gernot Warnke, PhD, is technical service manager for pharmaceutical R&D, both at Ashland Specialty Ingredients in Dusseldorf, Germany. Corresponding author Tom Watson is a global product manager at Pall Corporation, 5 Harbourgate Business Park, Southampton Road, Portsmouth, UK PO6 4BQ; 44-23-92338185; tom_watson@europe.pall.com. Supor, Fluorodyne, and Ultipor are trademarks of Pall Corporation.