Travel in January turned out to be more of an adventure than many of us anticipated. Two events that BPI attends in January were in Washington, DC: Phacilitate’s cell and gene therapy conference (part of its Washington Leaders Forum) and the CASSS Well Characterized Biotechnology Products conference (along with two CMC strategy forums). These two events present topics that we see carried throughout the year, including FDA initiatives and current regulatory, quality, analytical, and manufacturing focus points. Organizers of both…
March 2016
Spotlight
Introducing a New Editorial Advisor Nanda Subbarao is a senior consultant with the Biologics Consulting Group specializing in analytical, stability, and quality systems for chemistry, manufacturing, and controls (CMC) with good laboratory and manufacturing compliance (GLPs, GMPs). She has assisted pharmaceutical and biotechnology organizations in evaluation of analytical methods and method validation for a broad range of products ranging from conventional drugs to well-characterized proteins and vaccines, from preclinical to commercial phases. Subbarao has been involved several times over her…
The Year of Data Integrity: 2015 Brought a Worldwide Focus on Training, System Design and Control, and Data Management
Each year, regulatory agencies from around the world focus on critical aspects of the pharmaceutical quality management system, bringing awareness to the industry and continuing to effect positive change. In the past five years, risk assessments, electronic records, and outsourced activities have been in the spotlight. As 2015 closed out, it was clearly the year of data integrity. In March 2015, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) published its GMP Data Integrity Definitions and Guidance for Industry,…
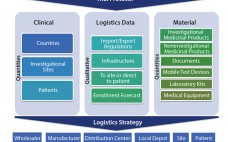
Clinical Supply Chain: A Four-Dimensional Mission
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (1). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers. The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application…
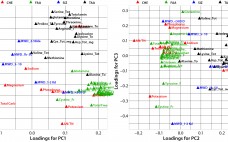
Multivariate Analysis of Biological Additives for Growth Media and Feeds
Biological additives such as yeast extracts and peptones are commonly used in growth-media formulations for biopharmaceutical manufacturing. In spite of drivers encouraging companies to reduce variability in mammalian cell culture processes by using chemically defined media, many microbial and mammalian processes continue to use biological additives in their growth-medium formulations and/or feeds. According to Sheffield Bioscience (Kerry, Inc.), at least six of the top 10 licensed mammalian-cell– derived biotherapeutic products are manufactured using biological additives (1). During process development, it…
Alkyl Mono- and Diglucosides: Highly Effective, Nonionic Surfactant Replacements for Polysorbates in Biotherapeutics — a Review
Many biotherapeutic proteins are naturally subject to aggregation. The clinical consequences of protein aggregation can be dramatic, not only affecting bioavailability and pharmacokinetics, but in extreme cases dramatically altering pharmacodynamics as well. Of equal or perhaps more importance is that aggregation is a principal source of unwanted immunogenicity in biotherapeutics. Aggregation-induced neutralizing antibodies and/or anaphylactic reactions are serious and growing US and European regulatory concerns. So they will have significant and growing influence on the future development and regulatory approval…
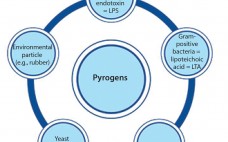
Detecting the Broad Spectrum of Pyrogens with the Human Whole-Blood Monocyte Activation Test
In the early 20th century, some patients injected with the drug Salvarsan experienced febrile reactions due to contamination of the drug’s distilled water. That incident (involving the first effective treatment for syphilis) prompted not only the widespread use of injectable drugs, but also the need for pyrogen control. Pyrogens constitute a heterogeneous group of microbial and nonmicrobial substances that include those derived from Gram-negative and Gram-positive bacteria such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), respectively, as well as particles…
Factors Affecting Sterile Filtration of Sodium-Carboxymethylcellulose–Based Solutions
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (1–3), serves as a suspension aid (4), and stabilizes emulsions (5). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (6, 7). CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree…
Insulin in Demand: Engineering a Facility to Serve Local and International Markets
Discovered in 1922 at the University of Toronto (by Frederick Banting and John James Rickard Macleod), insulin has been produced from animal extract, primarily cattle and pigs. Following Frederick Sanger’s work on insulin sequence in 1951, Dorothy Hogkin published in 1969 the three-dimensional structure of insulin. This breakthrough led to many developments and applications of recombinant DNA for the production of insulin, human first and then analogs. The significant increase in the diabetic population — especially in BRICS countries (Brazil,…
Opportunities in Latin America Beyond the Olympics
Olympics fans aren’t the only ones turning their eyes toward Brazil. According to JLL’s fourth annual Life Sciences Outlook, the 2016 Summer Games host nation is also one of the world’s top 10 “Global Clusters to Watch,” thanks to its proven capacity for medical device manufacturing and a growing healthcare market. Increasingly, Latin America overall has been on the radar for life sciences companies seeking greater operational efficiency and opportunity, expanded market access, promising demographics, lower-cost land and labor, and…