We might not associate the jazz queen Ella Fitzgerald with 21st-century gene-based therapies, but the First Lady of Song was on to something back in 1939 when she sang “’T’Ain’t What You Do (It’s the Way That You Do It).” Although demonstrating the safety and efficacy of gene-based therapies in rigorous clinical trials is essential for gaining product approval from regulators, doing the bare minimum is insufficient. The way that such products are produced also matters. Manufacturing processes and protocols…
Wednesday, June 10, 2020 Daily Archives
Anticipating Cell-Line Challenges to Drive CMC Readiness
Development of a safe and high-quality Chinese hamster ovary (CHO) cell line is of paramount importance for the chemistry, manufacturing, and controls (CMC) portion of studies that support investigational new drug (IND) applications (1, 2). Desirable attributes of a CHO cell line include its ability to produce high titers of biotherapeutic proteins facilitate quick recoveries and selection processes maintain phenotypic and genetic stability throughout in-vitro aging of a culture. A CHO cell line also should be scalable to high-capacity culture…
Writing the Future of Biologics to Discover and Optimize Antibodies
With our unique DNA writing technology, Twist Biopharma is accelerating the way our partners discover and optimize antibody therapeutics. We have decades of synthetic antibody library design and selection expertise and have the ability to write any DNA sequence to enable faster, better discovery and development. Using our precise, rational, expertise in library fabrication, we can create libraries in a fundamentally different way that allow us to address tough targets, e.g. GPCRs. Since we are a DNA product company, we…
Discover, Develop, Deliver
Astrea Bioseparations is the only adsorbent supplier that can discover new affinity ligands designed to bind selectively to a molecule of interest or specific impurity, develop efficient purification adsorbents and downstream methods, and deliver industrial-scale adsorbents (up to 1,000-L batch sizes) as loose slurry or in good manufacturing practice (GMP)-ready columns. With over 30 years of experience in development of affinity products and design and manufacture of new custom adsorbents, Astrea Bioseparations is a world leader in its field. The…
Retrofitting Your Bioreactor to Enhance Stirring Processes: Replacing Old Agitators with State-of-the-Art Magnetic Mixing Technology
A key challenge for companies involved in drug development is to meet the highest standards of sterile design and reliability. In this context, magnetic mixers offers many advantages for aseptic stirring processes compared to mechanically sealed agitators. ZETA not only supplies magnetic agitators for new bioreactors, but also supports its customers through the entire retrofitting process, from feasibility studies at the beginning to full process qualification and validation at the end. “Combat the risk of batch contamination in bioreactors and…
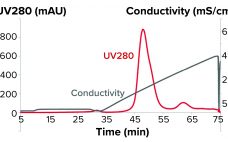
Reduce Downstream Processing Costs for MAbs By Switching to a Two-Step Platform
Downstream processing operations make up to 80% of the total costs for processing biotherapeutics. Given the current drive to reduce downstream costs, chromatographers and process engineers will need to streamline processes. Herein, we describe the benefits offered by using Tosoh’s two-step process for purifying monoclonal antibodies (MAbs) and compare that method with the standard industrial process. By combining high-performance protein A capture and a single polishing step on salt-tolerant anion-exchange resin, Tosoh’s approach can reduce downstream costs by 45% and…
Process Intensification of Viral-Based Vaccines: Where Are the Bottlenecks?
In the current coronavirus pandemic, the ability to scale up and produce viral-based vaccines (attenuated viral vaccines, inactivated viral vaccines, and viral vector vaccines) quickly and in large quantities has never before been more relevant. For viral-based vaccines that can be produced by adherent or suspension cell culture, process intensification — in which cell culture, for example, is optimized to produce higher viral titers using the same process equipment — offers a strategy to produce larger numbers of doses in…
Ask the Expert: Emerging Trends in Drug Assembly and Packaging
Thomas Gabriel (director of strategy and business development at Fujifilm Diosynth Biotechnologies, FDB) emphasized patient agency in his 15 April 2020 “Ask the Expert” presentation on innovations in finished-goods solutions. Facilitating self-administration significantly benefits patients with chronic conditions, and new delivery technologies are making drug products increasingly easy to handle and with increasingly accurate dosing. Gabriel explored how single-use autoinjectors and prefilled syringes, needle shields, on-body delivery systems, and digital monitors are improving drug-delivery safety and efficacy while enabling patients…
Ask the Expert: Improving Host-Cell Protein Detection By Enriching Mass Spectrometry Samples
Enzyme-linked immunosorbent assays (ELISAs) remain the industry standard for process monitoring and lot-release testing for host-cell proteins (HCPs), but researchers need orthogonal tests to confirm that an ELISA is fit for purpose. On 22 April 2020, Eric Bishop (vice president of research and development at Cygnus Technologies) delivered an “Ask the Expert” presentation about his company’s solution: mass spectrometry (MS) preceded by a novel Antibody Affinity Extraction (AAE) technique. Bishop explained that an AAE step can magnify a sample’s HCP…
Virtual Audits: A New Reality in the World of COVID-19
The novel coronavirus disease 2019 (COVID-19) pandemic has every industry seeking out ways to accomplish time-sensitive activities using a number of virtual approaches. This is certainly true in the biopharmaceutical sector, in which good manufacturing practice (GMP) audits are required to manufacture medicinal drug products for human use. Examples include supplier/vendor audits, mock inspections, and preapproval and prelicense inspections (PAIs and PLIs) conducted by sponsors and regulatory authorities. Auditors usually perform such activities on site and only sometimes remotely. In…