Orkila is pleased to announce a new exclusive partnership with Capsule Delivery Solutions, part of Lonza Pharma & Biotech, allowing Orkila to promote their whole Capsugel® capsule range in Egypt. “This agreement is an important part of our business strategy to drive growth and streamline our channel to market,” said Philippe Azzi, Capsule Delivery Solutions’ Territory Sales Manager for Lonza. “By leveraging Orkila’s extensive logistics network and deep industry expertise, we will be able to serve our Egyptian customers faster…
Friday, March 23, 2018 Daily Archives
March From the Editor
Are you protecting your trademarks? Are you sure? You’ll notice that BPI doesn’t use registration marks (e.g., ™ and ®) anywhere but in advertisements and the occasional “advertorial” piece. Ever wonder why? According to the official stylebook of the American Chemical Society, which is the basis of our “house style,” they are entirely unnecessary. And editors everywhere dislike the impression they give as well. On page 157 of the ACS stylebook, trademarks (“brand names”) are defined as adjectives that describe…
March Spotlight
Welcome New Editorial Advisor Kavita Ramalingam Iyer is associate director and product lead of GRACS-CMC for vaccines at Merck Sharp & Dohme Corp. She received her PhD in biotechnology from Anna University (India) and completed a postdoctoral fellowship at the University of Minnesota (focusing on antibody engineering and synthetic biology) before joining Merck in 2008. Kavita has over 10 years of pharmaceutical industry experience leading chemistry, manufacturing, and controls (CMC) development; manufacturing; establishment of good manufacturing practice (GMP) facilities; technology…
Back to Basics for Biotech: Driving a Culture of Quality and Compliance with Practical Communication Techniques
The robust regulatory environment surrounding biotechnology and bioprocessing demands a comprehensive current good manufacturing practice (CGMP) culture of quality, compliance, and absolute adherence to policy. Employees need to be engaged in their work, with a laser focus on meeting stringent specifications and operating under tight controls. A misstep in quality or compliance can lead to hefty fines, legal concerns, regulatory retaliation, and reputational damage. Communication and stakeholder engagement are critical to aligning organizations and driving the right culture in highly…
A Two-Step Purification Process: Application of HIC Membrane Chromatography in a Disposable 2,000-L Clinical Facility
Given paradigm shifts in the biopharmaceutical industry over the past decade, product development timelines are squeezed as the number of molecules entering clinical development continues to increase. Manufacturing facilities, especially those supplying clinical trial materials, have had to adapt to this trend. One popular approach is to have fully disposable equipment that allows for quick product changeover and flexible manufacturing capacity to respond to variable clinical demand. Although many facilities-related technologies exist to support that concept (e.g., disposable probes and…
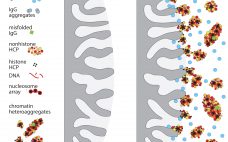
IgG Purification By Ultrafiltration: Time for Another Look
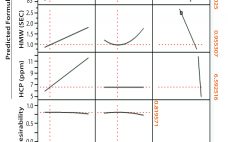
One of the early disappointments in development of immunoglobulin G (IgG) purification technology was ultrafiltration on membranes with 50–100 kDa cutoffs. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed that most host cell proteins were smaller than that. IgG was retained. Parallel concentration and buffer exchange could be performed going into a follow-on polishing step. These features made it an obvious candidate for initial capture, but it did not perform as hoped. Membrane fouling sabotaged its concentration–diafiltration potential, and prohibitive…
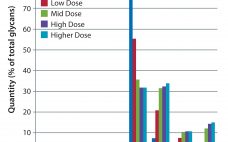
Enhanced Galactosylation of Monoclonal Antibodies: Using Medium Supplements and Precursors of UDP-Galactose, Part 2
In Part 1 of this report, we described our development of a high-throughput assay for analyzing monoclonal antibody (MAb) glycans and how we used it to evaluate the effects of medium supplements on galactosylation of MAbs produced by two different cell lines (1). This month, we examine galactosylation of a MAb produced by a third cell line. A discussion follows on the benefits of this high-throughput assay before we highlight the similarities and differences in galactosylation among the three MAbs…
Large-Scale Purification of Factor-IX: Comparing Two Affinity Chromatography Resins
Human clotting factor-IX (F-IX) is a glycoprotein that is essential for normal hemostasis (1). A deficiency of F-IX in human plasma is caused by an absence or functional mutation of the F-IX gene that expresses inactive F-IX in plasma. That leads to hemophilia B (“Christmas disease,” named after its first identified patient), a genetic disorder in which the blood-clotting cascade is disturbed (2, 3). The structure and amino-acid sequence of F-IX are similar to those found in other vitamin-K–dependent glycoproteins.…
Advanced Viral Clearance Study Design: A Total Viral Challenge Approach to Virus Filtration
Biologics derived from mammalian organisms have been accepted for therapeutic use for almost a century (1). However, these pharmaceuticals have the potential for contamination with pathogenic adventitious agents such as viruses. With cell-line–derived recombinant proteins, the viral risks commonly include viruses in the Retroviridae and Parvoviridae families (2). As patient safety and manufacturing facility suitability became significant concerns in the 1980s and 1990s, several industry and regulatory bodies reached consensus on how to approach the unique challenges of viral safety…
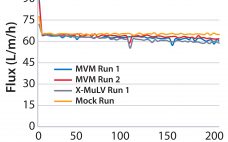
Evaluating Adsorptive Filtration As a Unit Operation for Virus Removal
Most recombinant monoclonal antibodies (MAbs) are produced by mammalian cells. Because biopharmaceuticals derived from mammalian tissue culture carry the risk of adventitious virus contamination, regulatory agencies expect risk-mitigation strategies to include validation of purification unit operations for their ability to clear viruses (1). Guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) describe how to prove viral clearance in downstream purification processes using an orthogonal approach (2). Viral log10 reduction values (LRVs) are…