Three-dimensional (3D) bioprinting is the newest addition to the regenerative medicine family. Now within the industry dedicated to providing more personalized drug products, this new additive-manufacturing technology has the potential to truly focus on individual tissue repair and replacement. In a short period of time, 3D bioprinting has been applied in studies using bones, blood vessels, composite tissues, vascular grafts, tracheal splints, cartilaginous structures, heart tissue (e.g., two-valve heart), and vaginal organs (1). View the full article below – Login…
Manufacturing
BioPhorum Operations Group Technology Roadmapping, Part 2: Efficiency, Modularity, and Flexibility As Hallmarks for Future Key Technologies
For a complex biopharmaceutical industry, setting out to forecast future technologies must involve considering how such technologies will be used. In the first article (1), I discussed why there was a need to develop a technology roadmap for the biopharmaceutical industry and the trends shaping its future: namely, the introduction of new product classes, the continued growth of the biopharmaceutical market, pressure to reduce costs, and uncertainty in approval and sales of new products. Herein I discuss the technology roadmap’s…
Buffers in Biologics Manufacturing
Biotechnology has enabled commercialization of protein-based drugs including insulin, growth factors, blood factors, and antibodies. Production and purification of such biologic products require different buffers for pH control and stabilization of reactions in different steps during biomanufacture. These processes include cell culture production (the “upstream” phase), purification (the “downstream” phase), and a final phase in which excipients are introduced to the drug substance to create a drug product (“formulation and storage”). In upstream processes, buffers are primarily used for their…
Improving Protein Folding Control and Scalability Using imPULSE Mixing Technology
This webcast features: Anthony Hawrylechko, Director of Microbial Bioprocess, Cytovance Protein folding by dilution is a common approach used in the manufacturing of biologics derived from microbial expression systems. This typically involves the solubilization of a washed inclusion body preparation containing the concentrated product polypeptide with a strong chaotrope or detergent solution. The denatured, inactive product solution is then diluted into a combination pH and red/ox buffer solution. Within this environment, the molecular diffusion rates of chaotrope, buffer components, and…
Biopharmaceutical Fill and Finish: Technical and Operating Challenges for the Latest Formulations and Devices
Because they occur after two highly engineering, and science-driven phases of biomanufacturing – expression and purification – biopharmaceutical fill and finish processes have not received the respect traditionally that they deserve. Yet of all competencies associated with bringing biopharmaceuticals to market, fill and finish arguably are the most specialized. This eBook reports on the technical and operating challenges impacting the latest formulations and devices including: outsourcing, contamination, standardization (pre-filled syringes), lyophilization, and serialization. Get informed on the current state-of-the-art technologies…
Single-Use Fill and Finish: An Interview with NNE Pharmaplan
I talked with NNE Pharmaplan’s Kim Vincent Andersen (single-use technology and biotechnology specialist) and Niels Guldager (global technology partner in biotech) to discuss their experiences with client facilities that incorporate significant elements of single-use technology. In particular, they highlighted a recent project for Novo Nordisk involving a large-scale greenfield filling and inspection facility in Hillerød, Denmark. Find more detailed information about the project online at https://goo.gl/yp4LQh. And you can watch a video about it here: https://youtu.be/czwwgdt3CxI. A Case Study You…
Elastomer Stoppers: Working Toward Adopting an Industry-Wide User Requirements Specification for Particulate Levels
Two years ago, the companies involved in the BioPhorum Operations Group (BPOG) fill–finish community agreed that the quality of elastomer stoppers for vials was causing problems for biopharmaceutical manufacturers. So they deemed it to be a priority for the group. The problem is particularly pronounced for vial stoppers used in legacy products, which may have been on the market for several years. Many such medicines remain valuable for large patient populations. The stoppers used on legacy medicines are manufactured using…
Reducing Clinical-Phase Manufacturing Costs: Collaborating for Savings without Compromising Quality or Performance
In downstream purification of monoclonal antibodies (MAbs), the single greatest contributor to manufacturing costs is the expensive capture step typically based on protein A affinity chromatography. Almost since its introduction to bioprocessing, efforts have been made to reduce the cost of this step. Several alternative ligands have been promulgated as potential replacements for protein A, but they have proven difficult to adopt and scale up. Supplier companies have pushed for increases in capacity and economics, but those are always accompanied…
The First Single-Use Diaphragm Valve: Automated and Controllable Systems Increase Process Reliability
Single-use components and systems now are firmly established in the pharmaceutical and biotechnology industries. The trend toward simplified and flexible upstream and downstream plant design means that these components are becoming increasingly important — especially in biopharmaceutical production. In the past, the only available disposables were primarily tubes, fittings, and possibly filters. But the number of single-use systems has been increasing for a number of years now. It is hardly surprising that plant designers and operators now can rely on…
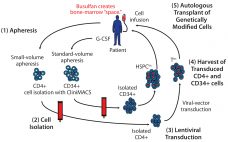
Cell-Delivered Gene Therapy: This Viral Vector Manufacturing Method Could Widen Its Applicability
Cell-delivered gene therapy is making an impact on a range of diseases (1–17). To date, successful treatments have generally been in conditions involving genetic deficiencies/abnormalities, for which introduction of a normal gene allele has been corrective (1–12, 18). Such an approach requires a vector containing the normal allele to overcome the mutant or lacking gene. The vector of choice for cell-delivered gene therapy is often a lentivirus that integrates and expresses introduced therapeutic genes in host target cells and their…