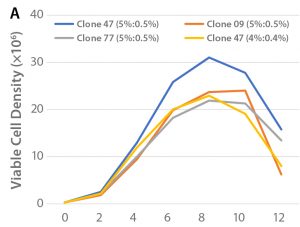
Figure 1: (A) Viable cell density over the culture period and (B) cell productivity on Day 12 of the tested clones in shakeflask cultures
Although the demographic of orphan therapies is small, making therapies for rare diseases available has a huge impact for the affected patients. Cooperation to expand capacity and expertise during process development and manufacturing for preclinical and clinical phase studies is one way to increase speed to market. This case study shares the work of GE’s Fast Trak Services team to help accelerate development of a process for cGMP production of material for toxicology studies. 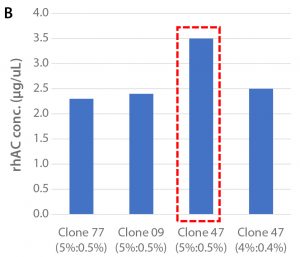 Frequent communication between the Fast Trak team and the client ensured transparency while protecting customer’s intellectual properties. GE scientists worked closely with Roivant Sciences to facilitate tech transfer, and a cGMP manufacturing process was developed. As a result, 400 grams of RVT-801 was produced for toxicology studies in 16 months.
Frequent communication between the Fast Trak team and the client ensured transparency while protecting customer’s intellectual properties. GE scientists worked closely with Roivant Sciences to facilitate tech transfer, and a cGMP manufacturing process was developed. As a result, 400 grams of RVT-801 was produced for toxicology studies in 16 months.
