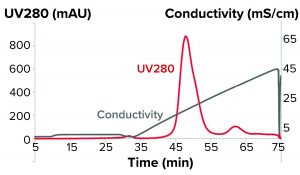
Figure 1: Bind-and-elute chromatogram of a monoclonal antibody purification on SkillPak 5 mL column with Toyopearl NH2-750F resin.
Downstream processing operations make up to 80% of the total costs for processing biotherapeutics. Given the current drive to reduce downstream costs, chromatographers and process engineers will need to streamline processes. Herein, we describe the benefits offered by using Tosoh’s two-step process for purifying monoclonal antibodies (MAbs) and compare that method with the standard industrial process. By combining high-performance protein A capture and a single polishing step on salt-tolerant anion-exchange resin, Tosoh’s approach can reduce downstream costs by 45% and increase production output by 58%.
Material and Methods
Resins and Prepacked Columns: Toyopearl AF-rProtein A HC-650F is a high-capacity protein A resin for MAb purification. This resin exhibits dynamic binding capacity (DBC) of 70 g/L at five minutes of residence time.
 The Toyopearl NH2-750F salt-tolerant anion-exchange resin is based on the Toyopearl methacrylate backbone and functionalized with primary amine groups. Toyopearl NH2-750F resin is ideal for intermediate purification of MAbs and other proteins. Impurities such as DNA, viruses, host-cell proteins (HCPs), and endotoxins are removed. Because of its relatively low pKa value (between 7 and 9), Toyopearl NH2-750F resin also removes MAb aggregates efficiently. With this unique feature, both polishing steps that usually are necessary to remove all impurities are combined in one flow-through polishing step.
The Toyopearl NH2-750F salt-tolerant anion-exchange resin is based on the Toyopearl methacrylate backbone and functionalized with primary amine groups. Toyopearl NH2-750F resin is ideal for intermediate purification of MAbs and other proteins. Impurities such as DNA, viruses, host-cell proteins (HCPs), and endotoxins are removed. Because of its relatively low pKa value (between 7 and 9), Toyopearl NH2-750F resin also removes MAb aggregates efficiently. With this unique feature, both polishing steps that usually are necessary to remove all impurities are combined in one flow-through polishing step.
All experiments were performed on SkillPak 5 mL prepacked columns. They are designed for fast method development and resin screening. The columns guarantee optimal performance and can be operated with commonly used low- or medium-pressure liquid chromatography systems. SkillPak columns are packed reproducibly and take into account the different compressibilities of resins, providing accurate representations of conditions found in full-scale columns.
Purification Protocols: Capturing with Toyopearl AF-rProtein A HC-650F Resin: Toyopearl AF-rProtein A HC-650F resin was equilibrated with 100 mM sodium phosphate (pH 7.0) and loaded with 20 CV (200 mL) of clarified cell culture fluid with 2 mg/mL adalimumab. The washing step was performed with 100 mM sodium acetate (pH 7.0) for 10 CV (50 mL). Elution was carried out with 100 mM sodium acetate (pH 3.0). The column was cleaned with 200 mM sodium hydroxide and then reequilibrated with sodium phosphate buffer. The flow rate was 204 cm/h for equilibration, washing, elution, and reequilibration; 150 cm/h for load; and 180 cm/h for clean in place (CIP).
Virus Inactivation: Eluate from the capturing step was held at pH 3.0 for one hour before it was adjusted back to pH 8.0 with 1 M TRIS. NaCl was used to adjust the eluate to the desired conductivity for the polishing experiments. The aggregate content after incubation at pH 3.0 was 1.05%.
Polishing with Toyopearl NH2-750F Resin: The protein A purified antibody was diluted to a concentration of 1 mg/mL and adjusted to conductivities of 20, 22.5, or 25 mS/cm with 20 mM Tris-HCl, pH 8. The column was equilibrated with different conductivities (20, 22.5, and 25 mS/cm — adjusted with NaCl) of the equilibration buffer. The sample was loaded for 40 CV (200 mL). Afterward, a washing step with equilibration buffer for 5 CV (25 mL) was performed. The column was cleaned with 500 mM NaOH. Flow rate during the entire process was 300 cm/h.
Analytical SEC: The load after a one-hour hold at pH 3.0 and the flow-through of Toyopearl NH2-750F resin were analyzed by size-exclusion chromatography (SEC) using a TSKgel UP-SW3000 column. The method can be found in Tosoh’s literature library, flyer F15L20A at https://www.separations.eu.tosohbioscience.com.
Results and Discussions
Process Development: In a previous study, we optimized capture of the antibody on the Toyopearl AF-rProtein A HC-650F column. After the capturing step, we introduced a virus-inactivation step by holding the eluate for one hour at low pH.
The first experiments on the salt-tolerant anion-exchanger Toyopearl NH2-750F resin were performed in bind-and-elute mode with a linear gradient at pH 8.0 to determine the necessary conductivity for the flow-through experiments (Figure 1).
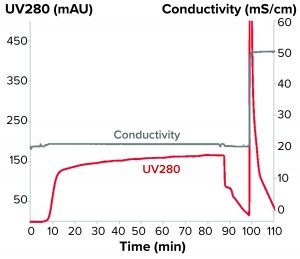
Figure 2: Flow-through chromatogram of a monoclonal antibody purification on SkillPak 5-mL column with Toyopearl NH2-750F resin
Because the monomer eluted first, we then adapted the method to achieve flow-through purification. For the flow-through experiments, we used conductivities between 20 and 25 mS/cm because the monomer elutes under those conditions whereas the aggregates remain bound to the resin. Table 1 lists purities and yields at three different conductivities.
To achieve the desired purity without a subsequent chromatography step, we chose 20 mS/cm conductivity for the platform design. Figure 2 shows the corresponding chromatogram.
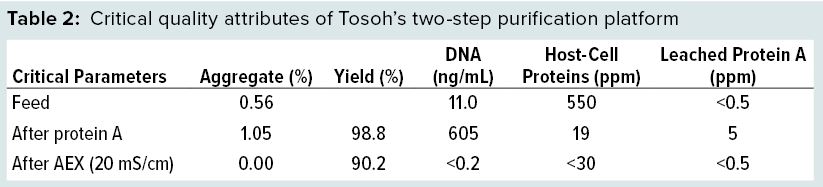
Purification Platform: Both chromatography steps were combined into one integrated process, including the intermediate low pH hold. Protein A had a recovery of 98.8%, and anion-exchange chromatography had a recovery of 91.3% (20 mS/cm), thus resulting in a total recovery of 90.2%. DNA, HCP, and leached protein A were removed to the limit of detection of the used assays (Table 2).
 Cost Analysis
Cost Analysis
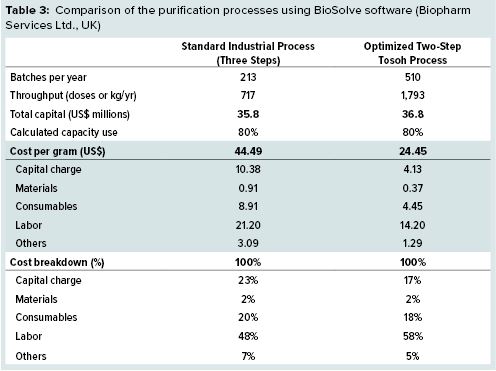
We used BioSolve (Biopharm Services Ltd., UK) bioprocess analysis software to compare the downstream costs of the optimized two-step process with the costs of an industry standard process published by BioPhorum Operations Group. As Table 3 shows, the optimized Tosoh process offers 45% lower costs per gram than the standard industrial process. And process times have been reduced by 58% because of the elimination of one chromatography step and higher flow rates on the Tosoh resins.
 Effective Cost Reduction
Effective Cost Reduction
Toyopearl NH2-750F is an effective anion-exchange resin for the removal of dimer and higher-order aggregates from MAb monomers. It is the ideal resin for streamlining purification processes of MAbs with high-performance protein A resins.
Tosoh Bioscience’s two-step antibody purification process (presented here using SkillPak prepacked columns) combines high recovery with low process costs and processing time. This platform offers a superior alternative to the industry’s three-step process. It can be scaled up easily to a pilot plant and eventually to manufacturing scale for increased productivity and profitability.
Patrick Endres is a senior laboratory specialist at Tosoh, Im Leuschnerpark 4, 64347 Griesheim; 49-6155-704-3745; patrick.endres@tosoh.com; www.tosohbioscience.de. Romain Dabre is a senior product manager at Tosoh, Im Leuschnerpark 4, 64347 Griesheim; 49-6155-704-3724; romain.dabre@tosoh.com; www.tosohbioscience.de.
Toyopearl is a registered trademark of Tosoh Corporation. SkillPak is a registered trademark of Tosoh Bioscience LLC in the United States.
