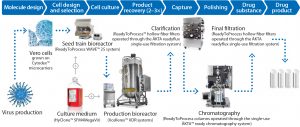
Figure 1: Process train comprises single-use equipment from GE Healthcare that help accelerate flavivirus vaccine manufacturing. Included systems are suitable for biomanufacturing of regulated products under various quality management systems. The systems are controlled through either GE Healthcare’s UNICORN™ or Schneider Electric’s Wonderware® system control software. To enable use of the systems in regulated environments, both programs are configured for use in a 21 CFR Part 11 and GAMP 5 compliant manner. All records are stored in a single, unalterable database, including results and extended run documentation. Specially trained and certified engineers perform onsite IQ/OQs and CCPs in accordance with cGMP, as well as provide on-site training for relevant personnel.
As with all viral vaccines, the complex nature of flaviviruses makes process development technically challenging. In addition, vaccine production can be both costly and difficult to scale to meet market demands. In egg-based vaccine production, for example, 100–300 vaccine doses can be produced from one fertilized hen’s egg. However, the eggs used for production need to be supplied from special pathogen-free chicken flocks, limiting availability of eggs and making vaccine production difficult to scale up. To meet the needs of preventive campaigns, including routine immunization and emergency response stockpiling, millions of vaccine doses would be required, making production both space- and resource-consuming.
