All bioproducts heading to commercialization go through a rigorous process that includes research and development, proof of concept, and validation studies. Furthermore, the fabrication process of a biological product is very different from that of a classical (synthetic small-molecule) pharmaceutical. That’s not only because of specific characteristics and requirements of the molecular entities involved, but also environmental considerations and specific issues related to human health and safety. Such differences are reflected in the cost drivers of approved manufacturing plants. Herein…
Search Results for: regenerative medicine
The Biotech Boom Down Under
Australia is a key global player in biotechnology investment for the Asia-Pacific region. The environment is characterized by outstanding research facilities, accelerating employment in the industry, uniquely Australian discoveries, and burgeoning alliances between Australian and international companies. Australia’s biotech companies are rapidly maturing. The country has 427 core biotech companies that are active in human therapeutics (48% of companies), agricultural biotech (16%), and diagnostics (14%) (1). Partnerships between multinationals and Australian companies and research organizations indicate growing international appreciation of…
Process Monitoring in Suspension–Adapted CHO Cell Cultures
Suspension-adapted Chinese hamster ovary cell (CHO-S) cultures are widely used in biotechnological production of recombinant proteins. In fact, such special cell lines have become the standard for this type of biopharmaceutical production (1). The reasons for that include their fast reproduction, high protein expression rate compared with other eukaryotic cells and, above all, the glycosylation patterns generated by the cells (2, 3). PRODUCT FOCUS: Animal cell products (recombinant proteins)PROCESS FOCUS: Production and product developmentWHO SHOULD READ: Process and cell culture…
Aurion Biotech secures $120m to advance cell therapy candidate
The funding will be used to develop Aurion Biotech’s first candidate, AURN001, a cell therapy aiming to treat corneal edema. Aurion Biotech is a clinical-stage biotech firm that is aiming to “restore vision to millions of patients” with its regenerative therapies. According to the firm, the raised funds will be used to file an IND to the US Food and Drug Administration (FDA) in order to begin clinical trials. Additionally, Aurion wants to submit an NDA to the Japan Pharmaceuticals…
Ask the Expert: Ultrapure Gelatin Can Optimize Your Excipient Screening for Vaccine Formulation
In his 28 May 2020 “Ask the Expert” presentation, Jeroen Geeraerts (business development manager at Rousselot) highlighted that, as of May 2020, 118 candidate vaccines for the novel coronavirus (SARS-CoV-2) had reached clinical or preclinical evaluation. Of those, 11 use inactivated and live–attenuated approaches, which require strong stabilizing agents to maintain vaccine potency. Geeraerts explained why inactivated and live–attenuated vaccines require stabilizing agents and why vaccine companies prefer pharmaceutical-grade gelatin for such applications. Next, Geeraerts described how his company’s X-Pure…
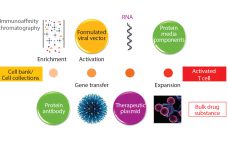
Cell and Gene Therapies Get a Reality Check: A Conversation with Anthony Davies of Dark Horse Consulting Group
As founder of cell and gene therapy (CGT) specialist firm Dark Horse Consulting Group in California, Anthony Davies speaks from a quarter century of experience including former positions at Onyx Pharmaceuticals, Syrrx, ZymeQuest, Serologicals, Geron Corporation, Capricor, and 4D Molecular Therapeutics — and he currently serves on the board of directors for TrakCel and the scientific advisory boards for Akron Biotech and BioLife Solutions. In his plenary address at the Phacilitate 2020 Leaders World conference (part of Advanced Therapies Week…
Development Approaches to Adenoassociated Virus Production
After many years of development, gene therapy is beginning to deliver on its promises in the clinic, in some cases with spectacular outputs. Those clinical successes also have led to an influx of funding and engagement from large pharmaceutical companies, thereby bringing the required financial support and expertise for late-stage clinical developments and product commercialization. Although many initial studies were confined to small patient groups and focused on a range of rare monogenetic diseases, new approaches to gene editing have…





 Welcome to BPI’s 15th-anniversary celebration...
Welcome to BPI’s 15th-anniversary celebration...