Moderator Dan Stanton, with Weichang Zhou, Jenifer Wheat, Roger Lias, and Jim Vogel Single-use technologies (SUTs) are now prevalent within bioprocessing, but does this spell the end of industry’s historic reliance on stainless steel and fixed facilities? This roundtable was formed to discuss the wealth of investment in single-use (SU) equipment and flexible manufacturing solutions by contract development and manufacturing organizations (CDMOs) over the past few years, pitting that against what looks like a resurgence in fixed-cost stainless steel plants…
Search Results for: niche disease
March Spotlight
Welcome New Editorial Advisor Kavita Ramalingam Iyer is associate director and product lead of GRACS-CMC for vaccines at Merck Sharp & Dohme Corp. She received her PhD in biotechnology from Anna University (India) and completed a postdoctoral fellowship at the University of Minnesota (focusing on antibody engineering and synthetic biology) before joining Merck in 2008. Kavita has over 10 years of pharmaceutical industry experience leading chemistry, manufacturing, and controls (CMC) development; manufacturing; establishment of good manufacturing practice (GMP) facilities; technology…
Assuring Multipotency of human Mesenchymal Stem Cells (hMSC)
Over the past decade, stem cell research has provided new avenues for deeper investigation into tissue repair and aging processes, as well as regenerative medicine methods. One of the major players in such research endeavors are mesenchymal stem cells (MSC), also known as mesenchymal stromal cells. MSC are typically found in bone marrow, adipose, placental, and umbilical cord tissues1 and are a type of adult stem cell. In vivo, these cells are headquartered in special microenvironments or “niches” in the…
Accelerated Development Through Strategic Analytical Partnerships
The analytical field for biologics has evolved greatly over the past 30 years, and the underlying growth has shifted from biopharmaceutical companies to contract research organizations (CROs). The global biopharmaceutical market is growing annually at >15%, making it the largest and consistently fastest growing segment of the healthcare industry with annual sales in excess of US$200 billion. Contract manufacturing organizations (CMOs) are expanding capacity by building new cost-efficient facilities, reflecting market demand. Many product sponsors are outsourcing, some even increasing…
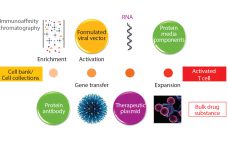
Manufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell Therapies
The concept of gene therapy is far from new, with initial studies performed over 20 years ago (1). However, in the past few years an explosion of interest in this area has gone beyond initial regenerative approaches using viral vectors. Interest is now moving increasingly into potential use of T cells modified using recombinant viral vectors for immunotherapy applications. Such therapies are based on using either adenoassociated virus (AAV) or lentivirus (1), both vectors being frequently generated through transient expression…
Managing Customer and Regulatory Expectations
Partnering with a contract development and manufacturing organization (CDMO) allows drug-product sponsors to turn fixed costs into variable costs. Market forecasting by pharmaceutical companies drives numerous decisions in development programs: sales-force resources, geographic resource distribution, and (of course) manufacturing planning. It is a widely accepted fact in the pharmaceutical industry that accurate forecasting is a challenge, especially for new drug launches. A number of models can be used to develop drug forecasts, but none of these models is perfect. No…
Outsourcing Biosimilars Development
A rapid increase in the number of companies working on development and registration of biosimilars has created a significant market for contract testing and manufacturing organizations (CTOs and CMOs) providing outsourced services specific to these products. Biosimilar developers turn to contract organizations when they lack either the internal capability or capacity for conducting certain work as well as when they require additional resources to bring products to market rapidly. A wide range of contract services are available, and each particular…
The Single-Use or Stainless Steel Decision Process: A CDMO Perspective
Decisions regarding whether and when to use single-use (SU) (disposable) devices or stainless steel (SS) equipment for biopharmaceutical manufacturing have been discussed for more than a decade. To date, no argument in terms of safety, cost-effectiveness, or operational efficiency is fully convincing to choose one technology platform or the other for all applications. Biopharmaceutical companies often do not have in-use data to make strategic manufacturing decisions. But one group has been significantly growing its expertise in use of single-use technologies:…
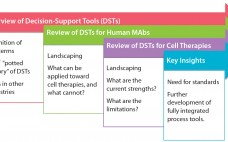
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…
Special Report: Turning Discoveries into Products — Developability Assessments and Highly Efficient Process Design
High costs and long timelines for biopharmaceutical development are cause for reflecting on how best to allocate resources from the earliest discovery stage through critical go–no-go junctures. With inputs ranging from science, engineering, and economics, the coined term developability becomes the synthesis of answers to such questions as How well does the target represent a disease state? Does manipulating that state bring about improvement? Does the molecule behave as expected in living systems? What can be done about the emergence of independent safety, toxicology, and/or immunogenicity warning signs? Can the molecule…