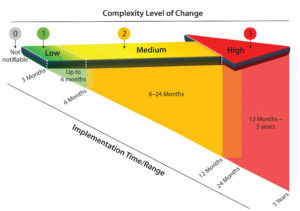
Figure 1: Time frames associated with implementing changes of varying complexity; arrows depicting that change complexity can be viewed as a continuum divided into discrete change levels. The diagonal indicates time frames required by recipients of changes before a recipient can qualify the change and accept the changed state.
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change on a customer difficult to understand: The regulatory environment is a murky picture, how drug manufacturers use supplier data is likewise opaque, and drug manufacturers do not have a uniform set of needs and expectations.
Thematically, those observations can be attributed to industry-wide deficiencies in communication and process standardization. Communication requires that customers explicitly inform suppliers of their regulatory, business, and technical drivers and expectations related to change notification as well as ways in which a supplier’s products are being used. Suppliers likewise must communicate changes clearly, promptly, and technically. Aligning all parties to a change notification process, to definitions of terms, and to clarified roles and responsibilities would facilitate communication and process standardization across the industry.
To address that issue, the BioPhorum Operations Group (BPOG) and the BioProcess Systems Alliance (BPSA) formed a team comprising contributors from 17 drug manufacturers and 12 suppliers. Their efforts led to the creation of a proposed practice to the industry, which is the subject of this article.
Our discussion herein presents elements of customer expectations, attributes of an effective change notification practice, categories of change, change and prechange notification content, and change notification workflow. A more comprehensive handling of these topics can be found in a white paper accessible through the BPOG and BPSA web sites.
The team’s objective is that this proposed practice to the industry be adopted by the vast majority of customers and suppliers in the single-use supply chain, to the point where parties reflect it in their own quality management practices and in formal business agreements.
Attributes of an Effective Change Notification Practice
A principal underlying concept is that material that contacts a regulated product or process (directly or indirectly) must be qualified sufficiently to preclude substances from being transferred to the product in quantities large enough to endanger human health, to bring about an unacceptable change to the composition of the product, or to affect the process. The elements of effective change notification listed below form the foundation of the proposed practice to the industry:
- Well-defined terms
- Defined lead times and timing for change milestones and deliverables
- A well-understood workflow
- Clear and aligned expectations
- High-quality data packages
- Content that anticipates customer needs
- Standardized report template
- Educated suppliers that continue to learn customers’ ways of working
- Common understanding of roles and responsibilities of suppliers and customers
- Single point of contact (SPOC) to facilitate and manage formal communication
- Ample opportunity and expectation for bidirectional feedback, as related to individual changes and to overall process effectiveness.
| Customer Expectations |
| Expectations that customers should have of suppliers who adopt this proposed practice to the industry are listed below. Without such rigor, there can be little confidence that the content of change notifications is grounded in a solid process and is traceable and defensible. |
Quality
|
Supply Chain
|
Manufacturing Practices
|
Technical
|
Categories of Change
The proposed practice to the industry was structured with an effort to align to other standards or guidance (3–8), while also recognizing that several relevant existing structures are not themselves aligned. Five levels of change are included in the proposed practice: emergency, 0, 1, 2, and 3. Figure 1 shows four of them.
The emergency change category acknowledges the reality of occasional changes that must be managed in an accelerated manner. Level 0 changes are non-notifiable changes that nevertheless must be managed within a supplier QMS. It is recommended that effective handling of level 0 changes be covered in a quality agreement.
Levels 1, 2, and 3 changes reflect increasing magnitudes of complexity, qualification, and impact on bioprocessing as well as implementation efforts associated with a change. The magnitude can be derived using risk assessment techniques (part of quality risk management in the proposed practice to the industry) and generally will be guided by a supplier’s statement of intended use and its customers’ typical use of affected products. In deriving a change level, the following should be considered: impact on form, fit, or function; size of study required to qualify the change; size of study required to demonstrate equivalence to the prechange state; and the potential impact on patients.
Content of a Prechange Notification
A prechange notification informs customers of an intent to initiate a relatively complex change. It provides them with an opportunity to ask for clarification or to request that certain information be part of an eventual notification package. A prechange notification should indicate, as applicable
- The reason for the change
- Affected products
- The anticipated date of the change notification
- The initial proposed qualification plan for the change (including timeline expectations and an outline of the anticipated qualification plan and report content)
- Recommendations for stock inventory
- Anticipated sample availability for end-user and customer evaluations.
Content of a Change Notification
A change notification provides a customer with the requisite information to understand the nature of a change, assess impact (or determine equivalence, if applicable), and manage the effect to maintain drug supply to patients. Several points should be considered when developing a change notification.
A recipient is a customer who receives the change notification. A thorough description of a change should include what the change is and is not, which allows customer to fully understand the change and be able to react to it internally. Rationale calls for contextualizing a change. Affected products are required to be listed in a notification for a customer to act on the change. The change level is determined by a supplier’s understanding of the complexity of a customer’s activities needed to qualify a change. Timelines should allow a customer to react to a change notification. Timing communication includes estimated dates for qualification completion, report availability, and implementation.
Identifiers enable a change notification to be traceable to the origin of a change. For an impact assessment in a change notification, the change originator should include a statement of potential impact of the change on its customers. The change originator also should provide information on how to request a qualification package, as applicable.

Table 1: Feedback mechanism to support effective change notification practices (SPOC = single point of contact).
A change notification also should include information on how a customer may receive samples of materials that represent the changed state, as applicable. For supply chain considerations, a change originator should inform a customer of how to know when it receives product that has incorporated the change. Finally, a change notification should describe last-time buy options and quantity restrictions to mitigate supply disruption risk, as applicable.
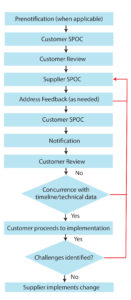
Figure 2: A representation of a generic change-notification work flow; black arrows indicate a change managed without issues between supplier and customer. Red lines indicate steps that are commonly associated with ineffective change management, steps that suppliers and customers should strive to avoid. (SPOC = single point of contact)
Change Notification Workflow
Perhaps the single most influential aspect of the proposed practice to the industry is the increased level of high-quality communication. This includes elements of timeliness, interactions between/among the correct individuals, and a right-first-time objective for communication content. The workflow in Figure 2 adds structure to this communication. Figure 2 calls for an SPOC to facilitate effective two-way communication. It further presumes a high-impact change is being managed, and thus contains prechange notification and a full complement of feedback steps. Changes of lesser impact naturally may not need the same level of attention to two-way communication.
Feedback
Continued and high-quality dialog among suppliers and customers helps to ensure alignment with a process and to each party’s expectations. Customers adopt communication strategies that vary with respect to resource intensiveness and quality of communication (Table 1).
Establishing a supplier and customer feedback strategy will help identify opportunities for improvement meaningful to both parties. Process misalignments and pain points as well as changes to expectations or needs should be topics for ongoing discussion.
Specific Actions to Begin Implementation
To implement the proposed practice to the industry, we suggest the following actions: First download the change notification template with its instructions and assemble a team to compare it with your current practices. What is similar? What is different? How could its principles be incorporated into your quality management system (QMS)?
Next, introduce the proposed practice to senior management who have the authority to agree to implement it. Develop a training and implementation plan for using the template and the associated work flow. Then implement an SPOC and request that your suppliers use the template and workflow. Finally, cite the proposed practice in your contractual agreements (contracts, quality agreements). Strive for high-quality communication with your suppliers to improve change notification quality over time.
As a support mechanism to the Industry, both BPOG and BPSA commit to housing and maintaining practitioner support materials, including an informational white paper, a change notification template, and a series of case studies. Those can be used to accustom yourself to the nature of various levels of change and to the documentation you can use to support change notification case studies of changes. We also will administer a discussion board and hold several online informational sessions.
Implementing the Practice
The team created this proposed practice to the industry to help the biopharmaceutical industry improve the efficiency and quality of change notification process. Implementation of this practice will help companies and regulators alike to ensure safe medicines. Widespread adoption of this practice will improve communication and education of both suppliers and manufacturers and will result in a more robust quality system. It also should shorten implementation times for changes because suppliers and manufacturers alike will gain a better understanding of one another’s requirements.
References
1 White T, Ott K. Management, Notification, and Documentation of Single-Use Systems Change Orders: Challenges and Opportunities. BioProcess Int. 13(9) 2015: 24–29.
2 Kline S, et al. Change Notifications for Single-Use Components: Criteria from an End-User Perspective. Pharma. Eng. 34(3) 2014: 1–8.
3 The International Pharmaceutical Excipients Council Significant Change Guide for Pharmaceutical Excipients, third revision. The International Pharmaceutical Excipients Council: Brussels, Belgium, 2014; http://ipec-europe.org/UPLOADS/IPEC_Significant_Change%20_Final_printing_09Oct2014.pdf.
4 USP <1195> Significant Change Guide for Bulk Pharmaceutical Excipients. US Pharmacopeial Convention: Rockville, MD.
5 Guidance for Industry: Deciding When to Submit a 510(k) for a Change to an Existing Device. Food and Drug Administration: Silver Spring, MD, 2016.
6 Guidance for Industry Changes to an Approved NDA (New Drug Applications) or ANDA (Abbreviated New Drug Application). Fed. Register 81, 2016: 52443–52444.
7 Postauthorisation Procedural Advice for Users of the Centralized Procedure. European Medicines Agency: London, UK, 2009.
8 ASME BPE: Bioprocessing Equipment. The American Society of Mechanical Engineers: New York, NY, 2016.
Corresponding author Jeffrey Carter is a strategic projects leader, Life Sciences, at GE Healthcare Life Sciences. Sabrina Restrepo is associate director, Sterile and Validation Center of Excellence, at Merck. Eric Isberg is director of life sciences at Entegris, and James Vogel is the founder and director of The Bioprocess Institute.
