As the debate continues over the high cost of pharmaceutical treatment options, the development of biosimilars continues to play a dominant role in that discussion and will be an important part of the solution. Biosimilar companies are working at a feverish pace to develop the next generation of follow-on products. Outsourcing to a growing group of contract development and manufacturing organizations (CDMOs) is a key strategy for savvy developers to accelerate their products’ launch.
Finding the right CDMO isn’t an easy task, however. Cost and capabilities continue to be key factors used to differentiate competitors in a crowded market, but biosimilar companies face a number of unique strategic challenges that must be considered as well. We interviewed companies that are sponsoring biosimilar candidates and CDMOs that manufacture such products to identify some challenges and find out how CDMOs are adapting to capture this portion of the biopharmaceutical market.
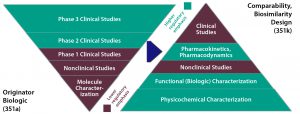
Figure 1: Totality of scientific evidence to characterize a biosimilar (size of segment = amount of effort required). Source: Calabrese L, Husni ME, Strand V. Biosimilars Part 2: Regulatory and Current Status. Biologic Therapies VI: Optimizing Therapies. The Cleveland Clinic Foundation: Lyndhurst, OH, July 2015; https://www.clevelandclinicmeded.com/online/webcasts/biologicsvi/biosimilars-2.
A Biosimilar Company Perspective
Biosimilar candidates follow a different development pathway from that of innovator molecules. A greater portion of time is spent upfront developing a process and characterizing a biosimilar molecule (Figure 1). Once that molecule is in clinical testing, its timeline to commercialization will be faster than that of an innovator molecule, and its likelihood of success in clinical studies is significantly higher. However, the ultimate cost of goods (CoG) must be low for a biosimilar to compete in the market with comparator molecules. The goal for both biosimilar and innovator companies is to get into clinical testing in the shortest amount of time using the least amount of resources. However, biosimilar companies distinctly aspire to minimize or even remove the clinical study commitment through robust process and product characterization — making certain aspects of their outsourcing development unique.
The number one factor in selecting a CDMO cited by all biosimilar companies we interviewed was commercial capability to manufacture their products. Once sponsors and CDMOs have invested the time to develop a relationship and perfect a process, the time available to ramp up to clinical comparative studies and commercial manufacturing is not long, so biosimilar companies do not want to switch to a different CDMO at that point. Such a change would require technology transfer activities and building a new relationship, additions to the overall timeline that they would rather prevent.
Commercial capacity does not always mean multiple large-scale tanks. With improvements in upstream process yield, some biosimilars can be produced commercially at smaller scales. But the ability to fulfill quality and regulatory requirements for commercial manufacturing are extremely important to biosimilar companies.
Cultural fit and flexibility from both a relationship and business standpoint also play a large role in CDMO selection. Biosimilar companies we interviewed stated that it is typically not the capabilities and/or technical aspects of the partnership that lead to failures; communication and corporate culture differences cause the biggest problems. Therefore, biosimilar companies are looking for an approach to program management that allows for adjustments and realistic timeline management.
Biosimilar companies have learned that it can take time to learn how to work with a CDMO partner, so investment in that relationship is important to the success of a given program. Once that investment has been made, biosimilar companies want to stay with the same CDMO and are likely to add additional programs as their pipelines progress. From a business standpoint, biosimilar companies know that development of a manufacturing process is highly variable and depends on large amounts of analytical data. Driving against the requirement for comprehensive analytics to support process development, accelerated timelines to phase 3 and commercial production require that a manufacturing process is locked in much earlier than for innovator programs.
Biosimilar companies prefer a model that is based on full-time employee (FTE) equivalents or highly flexible. This permits quick adjustments without requiring a number of change orders to a predefined scope. Biosimilar development typically requires a larger amount of consistency batches than innovator products do: about 10 batches, whereas innovator products need only three. As a result, biosimilar companies feel that CDMOs should be very competitive in pricing those batches given their much higher probability of success.
Additionally, a CDMO’s willingness to discuss exclusivity can be very important. None of the biosimilar companies we interviewed wanted a manufacturing partner that would be willing to work on the same molecule for a competitor. The ability to provide such exclusivity can be a game-changer in CDMO selection.
From a technical and capabilities perspective, the ability to perform advanced analytics, complex process characterization, and validation activities is an important factor for biosimilar companies. Given the emphasis on product characterization, analytical laboratories play a pivotal role in biosimilar development. Showing biocomparability through robust process and product characterization can minimize or even remove clinical comparability studies. Two companies we talked to felt that there were very few CDMOs that could meet their needs in this area. Assays can be outsourced to an analytical laboratory, but to prevent resource and scheduling issues they would prefer that a CDMO provide such capabilities in-house.
Location plays a large role, but it is not as important as those factors above. Interaction between a biosimilar company and its CDMO will be frequent and high-level. Biosimilar developers often provide their own program oversight, dedicating 0.5–2 FTEs of internal resources to oversee a given program. The exact level of such oversight depends on the stage of a program and the number of concurrent programs in the works. Face-to-face and on-site interactions are ideal, but modern technology does allow for more seamless remote interaction.
Historically, biopharmaceutical companies have looked for local CDMOs. But biosimilar companies are finding that they need to be more flexible regarding location. Many CDMOs have suite space booked out two years in advance these days. With a number of biological products coming off patent in 2020, it is assumed that demand for that space will increase.
Cost: In general, biosimilar programs do not face the same research and development costs that typically are associated with innovator products. However, the upfront product and process characterization is much more significant and challenging because of the need to compare biosimilar and reference products. Additional costs come with the expectation of a greater number of consistency batches. The cost of clinical manufacturing is relatively similar for both types of product. The cost of materials, suite time, and resources do not change based on the type of program. But the ultimate CoG is a critical factor in the overall success of a biosimilar program.
One biosimilar company we spoke with has chosen to invest in its own manufacturing facility to ensure ultimate control over cost outcomes. By keeping manufacturing and analytical development in-house, such companies can fit the facilities’ capacity and scheduling to their own pipelines. Such a contributing factor to the ultimate CoG cannot be controlled using a CDMO. For companies without the option of building their own facilities, establishing good partnerships with CDMOs presents a viable solution.
A CDMO Perspective
CDMOs say that biosimilars make up about 10–20% of their overall business and client base. Although two out of three CDMOs we interviewed actively pursue biosimilar clients, they are hesitant to focus on biosimilar work as a core business offering. With increasing needs for more advanced characterization and analytical development, bioreactors larger than 2,000 L, and lower overall CoG, the biosimilar market is not ideal for most CDMOs’ current capabilities and business needs because of their already low profit margins. Nevertheless, such companies do see value and growth in the biosimilar market and are continuing to position themselves as its support system.
When we asked CDMOs to list the three most important factors that they believe biosimilar companies want from them, their answers were all similar. All CDMOs interviewed cited a track record with the FDA, advanced analytical capabilities, and technical capabilities. Only one company cited commercial capability as a necessity.
All CDMOs seemed to understand that final CoG manufactured — and therefore the pricing of their services — were particularly important to biosimilar companies. Given the already slim profit margins of CDMOs, competing on price can be difficult. However, biosimilar candidates tend to progress to licensure quickly and face a lower chance of failure. So the CDMOs we spoke with still felt that such programs are a valuable subset of their business because of the high probability of a commercial manufacturing engagement with biosimilar clients. The challenge is matching suite availability and the possibility of commercial production with tight timelines, sufficient capacity, and other resources needed to be commercial ready.
CDMOs believe that biosimilar experience is valuable. Developers can learn quality and analytical aspects from working on biosimilar development. For example, raw materials need to be treated from the beginning as if they would be used for commercial products. The timeline to provide clinical material in support of accelerated progression into phase 3 and commercial manufacturing creates a risk of mismatch between such timelines and the availability of some raw materials that involve long lead times.
Having the experience of working on several biosimilar programs can help biosimilar companies to navigate such challenges. As Edwin Beale (senior director of corporate development at Cytovance) noted, chemistry, manufacturing, and controls (CMC) are “always on the critical path, there is no room for delays, stage-gating, or error. Having biosimilar experience enables us to overcome potential challenges before they occur.”
Maintaining technological superiority is critical to competing within the biosimilar space. As science and technology improve the characterization of biosimilar products, regulatory authorities and biosimilar developers require CDMOs to implement new tools in their analytical development. Such advanced tools often can decrease product development cycle times and costs. So CDMOs continually improve their analytical development capabilities. Those process improvements are synergistic for CDMOs and designed to attract all customers, not just biosimilar companies. Despite continued innovation and expansion of analytical capabilities, CDMOs note that biosimilar companies’ demands for analytical capabilities are increasing.
Companies we interviewed say biosimilar companies typically engage a CDMO around phase 1.However, a number of biosimilar companies engage a CDMO earlier on, with an objective of leveraging their experience in streamlining biosimilar product development. Advantages to early engagement can include obtaining reference products for analytical characterization and help in developing a biosimilar cell line.
The common theme we discovered in interviewing CDMOs was how busy they were. Demand for CDMOs and suite space is surging. Although CDMOs are increasingly making improvements in their offerings and capabilities, they need to balance those investments against what is needed for all types of clients.
CDMOs can be a valuable resource for biosimilar companies: providing streamlined development, experience with manufacturing, and sometimes even providing the tools needed to begin a program efficiently (e.g., the cell line, itself). For small sponsor companies without the resources to build their own internal manufacturing capability, finding a good outsourcing partner is not only necessary but critical to the success of their programs.
The Whole Picture
All the companies agreed that strength in analytical and technical capability was extremely important for biosimilar development, as was the final CoG. And all the biosimilar interviewees referenced the importance of developing partnerships as a top factor necessary to the program success.
CDMOs considering the business model of attracting biosimilar candidates note these advantages to such programs: the speed to attaining a commercial product, the lower risk of failure, and loyalty from biosimilar clients once they have begun to work together. But the ability to manufacture such products commercially must be part of their planning — as well as the willingness to work exclusively.
Assessing necessary analytical capabilities also is important because many biosimilar companies believe that those could be improved among CDMOs. The intensive process characterization and validation activities required for biosimilar candidates also can provide an attractive addition to outsourcing businesses. Finally, the message is clear from biosimilar companies that the importance of flexibility and cultural fit cannot be overlooked by CDMOs looking for biosimilar development business.
Cassidy Cantin is a business development and program management consultant, and corresponding author Kristi Sarno is a senior consultant at Latham BioPharm Group, 2 Mill and Main Place #440, Maynard, MA 01754; 1-978-266-9151; ksarno@lathambiopharm.com; www.lathambiopharm.com. Companies interviewed include Momenta Pharmaceuticals, Pfenex, Mabxience, Cytovance Biologics, KBI Biopharma, and Catalent Biologics.

