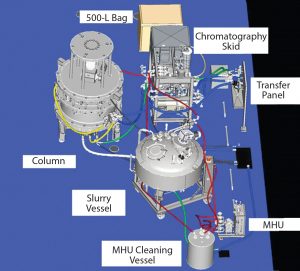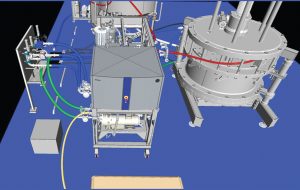
Figure 1: Modular equipment layout (A = 2-inch (235 LPM) HETP skid, B = 2 × 500-L buffer, C = 160-cm column, D = 1,600-L slurry vessel, E= 10,000-L buffer hold vessel, F = 800-L WFI break vessel)
To meet network demand for a commercial launch facility, Genentech (Roche) designed a new downstream train and built it within an existing building shell at the company’s Oceanside, CA, site. This downstream train included new technologies to allow for rapid technology transfer of different new products in the company’s drug pipeline. One technology that was pursued was the Axichrom column platform from GE Healthcare and associated column packing equipment to streamline column packing design. Here we focus on how a new approach to facility design provided flexibility, reduced capital investment, and mitigated process and start-up risks.
Flexibility
For a commercial launch facility dependent on rapid technology transfer, functionality needed to be adaptable to a variety of processing platforms as well as different resins. Flexibility balanced user requirements for current and future needs with the cost of implementing those requirements. Defining those needs up-front was imperative to constrain the design and the overall cost.
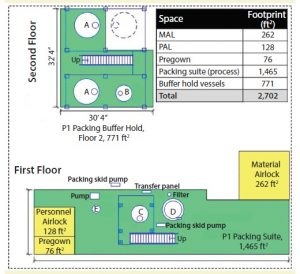
Figure 2: Stick-built equipment layout (A = 10,000 L buffer hold vessel, B = 2,000 L buffer hold vessel, C = 2,000-L slurry vessel, D = 1.8 m column, E = 200-L gradient vessel)
Design Approach: Open Ballroom Concept: For this project, a modular skid design was pursued as opposed to more traditional field-stick built approaches (Figures 1 and 2). The footprint needed to be as accessible or “open” as possible to accommodate uses that are both planned/ predictable and unanticipated. Portable slurry vessels make the open design possible by requiring no platforms, landings, or stairs. Process utility stations — transfer panels for buffers/caustics/water for injection (WFI) — and clean/steam-in-place (CIP/SIP) docking stations were kept at the periphery of the room, as were electrical, communication, and other utilities. Prebuilt skids were designed to be compact and portable (based on a modified standard system), which eliminated long flexhose runs and tightened up the overall footprint.
One drawback to modular and portable systems is the number of flexible hoses and set-up schemes required for both process and cleaning steps. From a planning and procurement perspective, the number of those hoses was greater than anticipated. The number had to be determined early to meet long-lead delivery dates, and the location had to be identified to allow for adequate personnel access and circulation space. The hoses also needed to be accounted for in parts washer loads and cleaning validation studies, depending on whether they would be cleaned in or out of place.
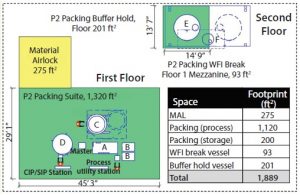
Footprint Use: The floorplan had to take into account flexibility for locating different operations. As an example of routine flexibility, the packing suite included a defined space for frit-storage units, column maintenance, and locations to anchor equipment to minimize movement during a potential seismic event. The company incurred additional costs in creating the long-term flexibility to pack columns in spaces adjacent to the purification and packing suites. To allow for column movement, integrated column lifter/mover/tugger technology was modified to allow docking to hitch-connection points built into the column framework. Other facility-fit modifications were required to make it possible to pack columns in multiple locations. Distribution piping and loops were provided to deliver process solution to use points in the purification suite via transfer panels that were field installed. This feature allowed all packing equipment to be used in the packing process, including a slurry vessel, a height equivalent to theoretical plate (HETP) skid, and a media-handling unit (MHU).
Future Flexibility: Future space was allocated for a personnel airlock and air-handling unit (AHU) to accommodate the possible need to separate the packing area from downstream chromatography operations. This additional footprint was set aside as unused space and thus represented an additional cost. But it offset the risk of future disruptive retrofitting to ongoing current good manufacturing practice (CGMP) operations. Setting aside this space provided additional room for process equipment (e.g., disposable bags) for packing operations and full chromatography operations (if required). In the even that the space is someday used for chromatography, then field piping and transfer panels would be required in the future.
Equipment Design and Selection: Requirements for Single-Use and Stainless Steel Systems: With the added footprint, the HETP/chromatography skid design was provided with sufficient inlets for both bags and connections to fixed vessels. The design offered capacity for 6 × 500-L disposable bags and accommodated flow rates up to 40 L/min (LPM), with connectors so that they could be aseptically docked to the chromatography skid using caustic. Modifications included a 1-inch outlet (with polypropylene support and elbow) and braided silicon tubing to ensure that pump cavitation (insufficient net-positive suction head) rather than collapse of tubing would be the limitation. The bag outlet was terminated with a sterile connector, and the entire bag assembly was gamma-irradiated before delivery. A 10,000-L buffer hold vessel, 10,000-L 0.5M NaOH vessel, and 800-L process water break vessel were provided.
Skid and Pump Sizing: During conceptual design, the company decided to minimize the footprint of the buffer hold space and to preclude additional purchase of buffer preparation vessels by implementing in-line dilution (ILD). The following requirements provided a sizing basis to select the appropriate pumps:
- 160-cm column diameter and 2-inch piping skid
- 5× ILD and process flowrate of 100–560 cm/h or 6.8–188 LPM (minimum flow 6.8 LPM, governed by four parts to one part for ILD)
- 5× ILD and unpacking flowrate of 30–300 cm/h or 2–101 LPM (minimum flow 2 LPM, governed by four parts to one part for ILD)
- Skid sanitization velocity of 7 ft/s (225 LPM)
These requirements represented a total pump operating range of 2–225 LPM. The wide range of flow rates required drove selection of two high-turndown diaphragm positive-displacement pumps, one sized for 1–83 LPM and the other 8–333 LPM.
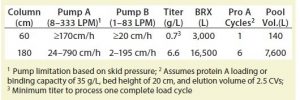
Facility Fit and Impact on Chromatography-Skid Design: A system with high turndown allows flexibility for different process platforms and scales. For example, a broad range of bioreactors from 3,000 to 16,500 L could be used to process titers ranging from 0.7 to 6.6 g/L with column diameters from 60 to 180 cm and 1–6 cycles (Table 1).
Use of disposable bags for small-volume low-throughput processing could be combined with stainless vessels using the same skid system. This design feature allowed for a wide range of processing conditions to be achieved. For a full range of chromatography operations, the skid was designed with eight inlets, four outlets, and all necessary instrumentation (e.g., UV, pH, conductivity, and air sensors).
The flexibility described above came at minimal added cost and was intended to meet needs if the network supply chain projections changed over time. To that point, the skid was purpose built for column packing operations, and integration into the existing facility would take additional piping, automation, and configuration modifications.
Automation Platform: Chromatography Functionality: An equipment design capable of both packing and chromatography operations at high pump turndowns was complemented by a Delta V automation platform (from Emerson) capable of the same flexibility. The software package included a full library of software objects. Phase logic and recipe structure used for traditional chromatography steps were combined with packing, unpacking, and priming phases in a hybrid structure.
At a strategic level, prebuilt chromatography functionality could be leveraged as a template for modular facilities across an entire network if needed for standardization and rapid implementation. As an example, UV is not used for column packing, but a phase is provided that incorporates a UV watch command and sequences to collect eluted product from the chromatography column (Figure 3).
Chromatography Resins and Recipe Structure: In addition to different operational modes, the automation system allowed for use of a range of compressible and incompressible chromatography resins without future code changes or recipe build. By minimizing skid modifications, all custom inputs and outputs (IOs) were incorporated into the base automation system. With those changes accounted for, the company implemented a recipe structure with formulas and parameters for each resin type anticipated for future technology transfers. That was combined with facility-integrated code (e.g., flowpaths from caustic systems, buffer hold, and process water). Flexibility for different packing conditions was provided by enabling bed detection for compressible (by pressure) and incompressible (motor current) resins upfront with the system vendor.
Reduced Capital Investment
A capital project in which cost, schedule, and quality are paramount requires an appropriate level of due diligence for the scope and selection of equipment required. A review of an existing stick-built packing suite (Figure 2) provided a recent benchmark from which to make strategic decisions regarding column technology as well as number and type of supporting equipment and vessels.
Prebuilt Skids Compared with Field Stick-Built: Construction Coordination and Field Contractors: Use of compact, prebuilt skids and vessels helped reduce the need for coordinating field construction. Although this saved contractor and construction management time, the price from the vendor included passivation, weld documentation, seismic calculations, and factory acceptance testing (FAT) costs. The result was a higher unit cost (compared with a line-item cost for stick-built components), but an overall cost savings given that field passivation, field welds, piping, platforms, and installation costs were significantly reduced.
Change Orders and Fees: Significantly fewer change orders and redesign efforts were needed because the vendor provided a standard system that could be modified with user input. Engineering design support also was reduced. But one potential drawback was the considerable amount of up-front user definition as a result of purchase, build, and delivery being scheduled early in the project. Flexibility to accept vendor standards also was required, while specific design changes were implemented, albeit with some difficulty given the design team was not colocated.
Equipment Design, Selection, and Cost: Process Equipment and Columns: The Axichrom column technology simplified equipment design, selection, and operation. Fewer standard buffers were required to pack and unpack each column. Early in the project, the company decided to pack and unpack in bactericidal solutions (caustic) and to conduct HETP tests using those same solutions. For most resins, that meant that existing caustic preparation vessel could be used if the loop was extended to the new packing suite. Those factors helped reduce the number of buffer hold vessels from three to one. The company standardized the size of the chromatography columns at 160 cm, thereby reducing the number of redundant purchases. But that came at a higher cost because each column was more expensive than the existing site standard.
Supporting Equipment: Transfer panels were used in place of multivalve arrays. The cost of supporting equipment was minimized because of reduced field piping, vessels, and IOs. But added costs were incurred to provide packing in the adjacent production suite, which required field-run piping, valves, IOs, and transfer panels.
Footprint Reduction: Equipment selection greatly affected the amount of space required. Given the lack of fixed platforms, field-run piping, and number of process and supporting equipment, facility footprint here was reduced by ∼30% from ∼2,700 to 1,900 ft2. Figures 1 and 2 show layouts for each packing suite (note that drawings are dimensional and comparable from a scale perspective).
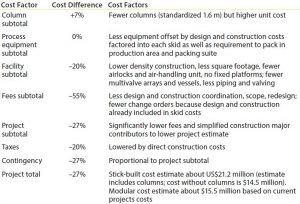
Cost Estimates: A total savings of US$5.7 million was realized compared to an existing column-packing suite. This number included the cost for existing facility column technology and the Axichrom technology for the new facility. It did not account for automation costs in either scenario. Although cost per square foot was not used to determine the total project cost, the reduction in footprint (30%) was comparable with the estimated cost reduction (27%) in Table 2. Major factors for cost savings were reduced fees and construction costs as a result of a simplified floorplan and prebuilt systems. An added benefit was that equipment could be delivered in advance of mechanical completion and automation delivery.
Automation Platform: Although automation was not incorporated into cost estimates for this evaluation, a net savings can be expected from it. In general, the column packing suite was almost considered a “segregated” system, with requirements defined upfront and known issues resolved early with the automation vendor.
A substantial amount of detailed automation scope resided in the vendor boundary. Physical layer and lower-level procedural code was prebuilt for all major packing, unpacking, and priming operations. The project was responsible for integration of partner units and flow paths (buffer hold, WFI, and caustic system) and CIP/SIP of the slurry vessel. Although the scope and operational requirements could be defined up-front, some challenges resulted from the column packing being considered as segregated. The effort for facility integration and recipe-build was significantly underestimated, which compromised software testing and schedule.
Code Changes: Design changes that affected automation were defined, managed, and resolved ahead of FAT. These changes then were tested with the full system in an operational setting at the vendor site before shipment. Punchlist items were resolved, and upon arrival to site, no additional code changes were made within the vendor boundary.
Commissioning Timelines: As a result of clearly defined scope and changes needed, a relatively straightforward commissioning plan could be developed with a reduced scope. In terms of cost and testing time, that allowed the project team to conduct commissioning against a subset of tests that focused on unique resins that were not prepopulated with parameters from the resin manufacturer.
Mitigation of Process and Start-Up Risk
A rapid technology transfer program requires reliable, consistent, and scalable systems and processes. To that end, the project needed to deliver a high-quality product on schedule but without time-consuming start-up, commissioning, and column packing studies at the full production scale.
Prebuilt Skids Compared with Field Stick-Built, Schedule Risk: Prefabricating the majority of skids and system off-site allowed the packing suite delivery to be decoupled from the bulk of facility construction activities. The result was that equipment was delivered from the vendor about six months ahead of mechanical completion, thus preventing construction delays. That allowed the project team to test and fit mobile equipment outside of critical-path construction execution and coordination.
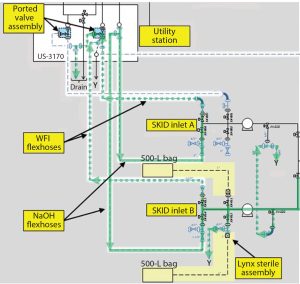
Equipment Design and Selection: Custom 3D Model: In addition to facilitating overall construction efforts, a detailed three-dimensional (3D) model allowed details for the column-packing system to be defined in advance of late-stage facility design and ordered from an automatically generated list. Importing vendor files into the model facilitated optimal equipment arrangement, with defined flexhose lengths and custom-designed ported valve assemblies.
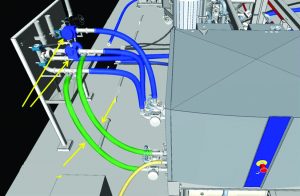
Without the model, detecting components clashing and providing access to drain points and column maintenance spaces would not have been possible. Specifically, much of the auxiliary equipment connected to custom utility panels would have significant interference or simply not fit. Large equipment (e.g., columns, slurry vessel, and chromatography skid) was both heavy and mobile, so the arrangement had to be exact to allow for appropriate logistics and transport. This provision allowed for parallel operations (e.g., packing one column while disassembling another) to be executed without the risk of interruption. In addition, finer details such as fittings and manual drain valves could be ordered early to meet an accelerated schedule. Figures 4 and 5 show general arrangement details.
Sanitary and Closed Processing Design Concepts: Bioburden contamination is a process risk factor for commercial production and has a significant impact on batch throughput and product quality. With speed to launch being a factor, contamination could shut down operations and present delays to batch release. Early in the feasibility phase, it was decided to pack, unpack, and HETP test all chromatography resins in bactericidal solutions. All resins (except for protein A) would use 0.1 and 0.5 M NaOH for operations and HETP testing. Protein A would use a bactericidal buffer and a version of that buffer at higher ionic strength for the HETP pulse.
Another way to combat bioburden risk is to implement engineering design controls. Automated valves were included to improve the sanitary design by enhancing displacement of air at high points and maximizing drainability (at specified low points). The systems also were designed with a compact piping arrangement to eliminate dead legs. Further design modifications allowed for process piping to be cleaned at a velocity of 7 ft/s to remove air through automated valves located at system high points.
As much of the process as possible was rendered closed. This was achieved by using ported valve assemblies and sterile-to-sterile connectors that were used to “dock” equipment with 0.5M NaOH. Loop pressure from the caustic supply system was used as the driving force to clean piping and connections in both the forward and reverse direction (Figures 6 and 7) using the utility station. The slurry vessel also was designed for automated CIP and SIP. All connections to and from the vessel used radial sterile access valves or ported valve assemblies mounted to the vessel frame. Transfer to and from resin containers was not intended to be performed in a closed system. To account for this, the slurry vessel was designed to be mobile. This meant it could be transported to another cleanroom for open operations and decoupled from ongoing production if temporal segregation was not possible (due to a high production run rate).

Figure 8: Pilot lab equipment layout (A = 500-L bag mixer, B = 14/20 cm column, C = 1/4-inch chromatography skid, D = 100-L bags, E = viral filter cart, F = 500-L WFI break, G = 250-L bag mixer, H = 1-inch chromatography skid, I =250-L bags, J = ultrafiltration system, K = 50-L bag mixer, L = master, M = 200-L slurry vessel, N = slurry packing system, O = HCU, P = depth filter holder, Q = peristaltic pump, X = 40-cm column)
Scale-Down Equipment: Scale-down equipment was purchased in parallel for a new 400-L bioreactor-scale, pilot-plant facility. Requirements and design specifications (to address scale factors such as mixing, piping diameters, vessel geometry, pump selection, filter housings, and valves/instrumentation) were reviewed and finalized alongside large-scale equipment so that the equipment delivered on the need for comparability. A large-scale 40-cm column, 200-L slurry vessel, and 1-inch chromatography skid were delivered ahead of the large-scale projects mechanical completion date Figure 8 shows the pilot-plant arrangement.
The same positive-displacement diaphragm pumps were used as were valves and instrumentation, so that sequencing, flowpaths, and hydraulics were comparable between small and large scale. Other scale factors taken into account included vessel geometry, impeller design (for the vessel), piping diameters, and filter housing/bubble-trap size (for the skid), which could affect mixing uniformity for vessels and Reynolds numbers and extra-column dispersion for skids. Figure 9 shows 3D representations of equipment selected for both small and large scales.
Comparability Studies: Packing and unpacking studies were conducted six months before the first commissioning test and provided confirmation for conditions of those resins used in the first product to be transferred. All resins were packed to target bed heights, and factors influencing reproducibility were identified and resolved. Specific parameters such as bed detection for an incompressible resin and optimization of slurry concentration measurement tests were the main factors that required evaluation. With those items resolved, only hydraulic and automation sequences needed confirmation during commissioning.
Automation Platform: Code Comparability: With scalable equipment designed and delivered, a similar specification process was conducted for both large- and small-scale automation systems. To ease programming for laboratory technicians as well as facilitate long-term system maintenance, GE Healthcare’s Unicorn automation platform was chosen for the pilot plant with the Delta V system for the large-scale process. Even taking those differences into account, the main packing, unpacking, and priming sequences within the vendor boundary were driven by the same local programmable logic controller (PLC) and therefore were identical between small- and large-scale systems.
Vendor FAT Testing: To mitigate start-up risk, individual package FATs were conducted, culminating with a full-system FAT using Delta V code to interface with the local PLC. At the vendor facility, a 160-cm acrylic column was packed, unpacked with a production chromatography resin, and HETP tested multiple times. By conducting the FAT on live equipment, the team reduced risk to code within the vendor boundary by testing synchronization, sequencing, and completion to functional requirements. Small commissioning-type punchlist items were resolved (e.g., timing, added priming flowpaths, and parameter definitions) for automation scope. Specific to the equipment, hydraulics, pressure control, pump performance, pump tuning, turndown, and response time were evaluated for in-line dilution. For packing and unpacking operations packed-bed quality, pressure drop and unpacking volumes also were verified.
The project team faced challenges with a particular resin during pilot testing with unpacking in 0.1M NaOH: Bed collapse did not occur within a minimum volume. The vendor developed a new method, and that was tested on the equipment used for production during the FAT with the resin in question. The tests confirmed a successful unpacking procedure, which achieved the required volume to fit in the slurry vessel.
Prepopulated Parameters: All vendor resins were provided with prepopulated pack and unpacking parameters. Other resins not sold by the vendor had recipe parameters determined ahead of time in the pilot plant or in collaboration with the system vendor. In the latter case, the vendor provided significant technical support to develop a bed-detection method and parameter definition for a future incompressible resin used in a legacy process. With significant challenges overcome ahead of formal start-up, facility-integrated recipe troubleshooting could focus on the scope outside the vendor boundary, with the vendor assisting on nonroutine troubleshooting both in the field and remotely. That proved to be highly successful because the automation resource provided by the vendor developed the Delta V platform with end-user input so issues that could have been major hurdles in the field were overcome efficiently.
Corresponding author David Page was principal engineer, and Chris Krein is principal technician at Genentech (a member of the Roche group); 1 Antibody Way, Oceanside, CA 92056; dpage@kbibiopharma.com.