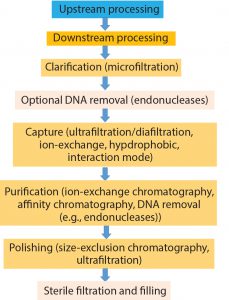
Gene therapy is the transfer of genetic material to a patient’s cells to achieve a therapeutic effect. Therapeutic DNA is largely delivered using viral vector systems based on adenoviruses (Ad), adenoassociated viruses (AAV), and lentiviruses (LV). With the application of such viral vectors as clinical therapeutics growing, scalable commercial processes (particularly for purification) are being investigated and optimized to best ensure that critical quality attributes (CQAs) are retained. Herein we review viral vector purification techniques and the effect of different characteristics of vector classes on the selection of optimal unit operations.
View the full article below – Login Required
Please log in to access this content.
References
1 Lukashev AN, Zamyatnin AA Jr. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochemistry (Mosc). 81(7) 2016: 700–708; doi: 10.1134/S0006297916070063.
2 Aiuti A, et al. Lentiviral Hematopoietic Stem Cell Gene Therapy in Patients with Wiskott–Aldrich Syndrome. Science 341(6148) 2013: 1233151; doi: 10.1126/science.1233151.
3 Cartier N, et al. Hematopoietic Stem Cell Gene Therapy with a Lentiviral Vector in X-Linked Adrenoleukodystrophy. Science 326(5954) 2009: 818–823; doi: 10.1126/science.1171242.
4 Biffi A, et al. Lentiviral Hematopoietic Stem Cell Gene Therapy Benefits Metachromatic Leukodystrophy. Science 341(6148) 2013: 1233158; doi: 10.1126/science.1233158.
5 dos Santos Coura R, Beyer Nardi N. Genetics A Role for Adenoassociated Viral Vectors in Gene Therapy. Genetics Mol. Bio. 31(1) 2008: 1–11.
6 Morenweiser R. Downstream Processing of Viral Vectors and Vaccines. Gene Ther. Oct. 2005: S103–S110.
7 Lyddiatt A, O’Sullivan D. Biochemical Recovery and Purification of Gene Therapy Vectors. Curr. Opin. Biotechnol. 9(2) 1998: 177–185.
8 Segura MM, Kamen A, Garnier A. Downstream Processing of Oncoretroviral and Lentiviral Gene Therapy Vectors. Biotechnol. Adv. 24(3) 2006: 321–337.
9 Ball PR, Gyepi-Garbrah I. Membrane Adsorbers as a Tool for Rapid Purification of Gene Therapy Products.
10 Burova E, Ioffe E. Chromatographic Purification of Recombinant Adenoviral and Adenoassociated Viral Vectors: Methods and Implications. Gene Ther. Oct. 2005: S5–S17.
11 Gao G, Vandenberghe LH, Wilson JM. New Recombinant Serotypes of AAV Vectors. Curr. Gene Ther. 5(3) 2005: 285–297.
12 Clark K, et al. Highly Purified Recombinant AdenoAssociated Virus Vectors are Biologically Active and Free of Detectable Helper and Wild-Type Viruses. Hum. Gene Ther. 10(6) 1999: 1031–1039.
13 Huyghe BG, et al. Purification of a Type 5 Recombinant Adenovirus Encoding Human p53 By Column Chromatography. Hum. Gene Ther. 6(11) 1995: 1403–1416.
14 Merten OW, Hebben M, Bovolenta C. Production of Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 13 (3) 2016: 16017. doi: 10.1038/mtm.2016.17.
15 Lusky M. Good Manufacturing Practice Production of Adenoviral Vectors for Clinical Trials. Hum. Gene. Ther. 16, 2005: 281–291.
16 Green AP, et al. A New Scalable Method for the Purification of Recombinant Adenovirus Vectors. Hum. Gene Ther. 13(16) 2002: 1921–1934.
17 Kamen A, Henry O. Development and Optimization of an Adenovirus Production Process. J. Gene Med. 6, 2004: S184–192.
18 Huyghe BG, et al. Purification of a Type 5 Recombinant Adenovirus Encoding Human p53 By Column Chromatography. Hum. Gene Ther. 6(11), 1995: 1403–1416.
19 Blanche F, et al. An Improved Anion-Exchange HPLC Method for the Detection and Purification of Adenoviral Particles. Gene Ther. 7(12) 2000: 1055–1062.
20 Peixoto C, et al. Purification of Adenoviral Vectors Using Expanded-Bed Chromatography. J. Virol. Methods 132(1–2) 2006: 121–126.
21 Vellekamp G, et al. Empty Capsids in ColumnPurified Recombinant Adenovirus Preparations. Hum. Gene Ther. 12(15) 2001: 1923–1936.
22 Konz JO, et al. Development of a Purification Process for Adenovirus: Controlling Virus Aggregation to Improve the Clearance of Host Cell DNA. Biotechnol. Prog. 21, 2005: 466–472.
23 Smith R H, Levy JR, Kotin RM. A Simplified Baculovirus-AAV Expression Vector System Coupled with One-Step Affinity Purification Yields High-Titer rAAV Stocks from Insect Cells. Mol. Ther. 17(11) 2009: 1888–1896; doi: 10.1038/mt.2009.128.
24 Urabe M, et al. Removal of Empty Capsids from Type 1 Adeno-Associated Virus Vector Stocks By Anion-Exchange Chromatography Potentiates Transgene Expression. Mol. Ther. 13(4) 2006: 823–828.
25 Zolotukhin S, et al. Production and Purification of Serotype 1, 2, and 5 Recombinant Adenoassociated Viral Vectors. Methods 28(2) 2002: 158–167.
26 Qu G, et al. Separation of Adeno-Associated Virus Type 2 Empty Particles from Genome Containing Vectors By Anion-Exchange Column Chromatography. J. Virol. Methods 140(1–2) 2007: 183–192.
27 Kaludov N, Handelman B, Chiorini JA. Scalable Purification of Adeno-Associated Virus Type 2, 4, or 5 Using Ion-Exchange Chromatography. Hum. Gene Ther. 13, 2002: 1235–1243.
28 Gao G, et al. Purification of Recombinant Adenoassociated Virus Vectors By Column Chromatography and Its Performance In Vivo. Hum. Gene Ther. 11(15) 2000: 2079–2091.
29 O’Riordan, et al. Scalable Chromatographic Purification Process for Recombinant Adeno-Associated Virus (rAAV). J. Gene Med. 2, 2000: 444–454.
30 Brument N, et al. A Versatile and Scalable Two-Step Ion-Exchange Chromatography Process for the Purification of Recombinant Adeno-Associated Virus Serotypes-2 and -5, Mol. Ther. 6(5) 2002: 678–686.
31 Zolotukhin S, et al. Recombinant Adenoassociated Virus Purification Using Novel Methods Improves Infectious Titer and Yield. Gene Ther. 6(6) 1999: 973–985.
32 Chahal PS, Aucoin MG, and Kamen A. Primary Recovery and Chromatographic Purification of Adenoassociated Virus Type 2 Produced By Baculovirus/Insect Cell System. J. Virol. Methods 139, 2007: 61–70.
33 Grimm D, et al. Novel Tools for Production and Purification of Recombinant Adenoassociated Virus Vectors, Hum. Gene Ther. 9, 1998: 2745–2760.
34 Clark KR, et al. Highly Purified Recombinant Adenoassociated Virus Vectors Are Biologically Active and Free of Detectable Helper and Wild-Type Viruses. Hum. Gene Ther. 10, 1999: 1031–1039.
35 Anderson R, et al. A Method for the Preparation of Highly Purified Adenoassociated Virus Using Affinity Column Chromatography, Protease Digestion and Solvent Extraction. J. Virol. Methods 85, 2000: 23–34.
36 Harris JD, Beattie SG, Dickson JG. Novel Tools for Production and Purification of Recombinant Adenoassociated Viral Vectors. Methods Mol. Med. 76, 2003: 255–267.
37 Smith RH, Ding C, Kotin RM. Serum-Free Production and Column Purification of Adenoassociated Virus Type 5. J. Virol. Methods 114, 2003: 115–124.
38 Auricchio A, et al. A Single-Step Affinity Column for Purification of Serotype-5–Based Adenoassociated Viral Vectors. Mol. Ther. 4, 2001: 372–374.
39 Sayroo R, et al. Development of Novel AAV Serotype 6 based Vectors with Selective Tropism for Human Cancer Cells. Gene Ther. 1, 2016: 18–25.
40 Davidoff AM. Purification of Recombinant AdenoAssociated Virus Type 8 Vectors By Ion-Exchange Chromatography Generates Clinical Grade Vectorstock. J. Virol. Methods 121, 2004: 209–215.
41 Vicente T, et al. Purification of Recombinant Baculoviruses for Gene Therapy Using Membrane Processes, Gene Ther. 16, 2009: 766–775.
42 Wu C, Soh KY, Wang S. Ion-Exchange Membrane Chromatography Method for Rapid and Efficient Purification of Recombinant Baculovirus and Baculovirus gp64 Protein. Hum. Gene Ther. 18, 2007: 665–672.
43 Scherr M. Efficient Gene Transfer into the CNS By Lentiviral Vectors Purified By Anion Exchange Chromatography. Gene Ther. 9, 2002: 1708–1714.
44 Yamada K, et al. Lentivirus Vector Purification Using Anion Exchange HPLC Leads to Improved Gene Transfer. Biotechniques 34, 2003: 1074–1078, 1080.
45 Segura MM, et al. Production of Lentiviral Vectors By Large-Scale Transient Transfection of Suspension Cultures and Affinity Chromatography Purification. Biotechnol. Bioeng. 98, 2007: 789–799.
46 Pettitt D, et al. Emerging Platform Bioprocesses for Viral Vectors and Gene Therapies. BioProcess Int. 14(4) 2016: S8–S17.
47 Rincon MY, Vandendriessche T, Chuah MK. Gene Therapy for Cardiovascular Disease: Advances in Vector Development, Targeting, and Delivery for Clinical Translation. Cardiovas. Res. 108(1) 2015: 4–20.
48 Kay MA, Glorioso JC, Naldini L. Viral Vectors for Gene Therapy: The Art of Turning Infectious Agents into Vehicles of Therapeutics. Nature Med. 7(1) 2001: 33–40.
49 Sheu J, et al. Large-Scale Production of Lentiviral Vector in a Closed System Hollow Fiber Bioreactor. Mol. Ther. Meth. Clin. Dev. 2, 2015: 15020.
50 Kallel H, Kamen AA. Large‐Scale Adenovirus and Poxvirus‐Vectored Vaccine Manufacturing to Enable Clinical Trials. Biotechnol. J. 10(5) 2015: 741–747.
Maya Fuerstenau-Sharp is a field-marketing manager at Sartorius Stedim (Göttingen, Germany). David Pettitt is an academic surgeon, CASMI research associate, associate at IP Asset Ventures, Ltd, and a doctoral researcher at the University of Oxford. Kim Bure is director of regenerative medicine at Sartorius Stedim. James Smith is a CASMI research associate and doctoral researcher in the University of Oxford’s Nuffield Department of Orthopedics, Rheumatology, and Musculoskeletal Sciences, and an associate at IP Asset Ventures, Ltd. Jamie Ware is a CASMI research associate. Corresponding author David Brindley is founder and academic director of CASMI’s Translational Stem Cell Consortium, senior healthcare translation research fellow in the medical sciences division’s department of paediatrics at the University of Oxford, Cooksey-Saïd fellow in healthcare translation at the University of Oxford’s Saïd Business School, honorary senior research associate at the University College London School of Pharmacy’s Centre for Behavioral Medicine, a research fellow in cell therapy commercialization at the Harvard Stem Cell Institute, and regenerative medicine regulation and risk management lead at the USCF Stanford Center of Excellence in Regulatory Science and Innovation (CERSI) in Stanford, CA.