Time to market, resource requirements, cost, and flexibility are key considerations in designing purification processes suitable for manufacturing biopharmaceutical products. Over the past decade, many advances have been achieved in disposable processing systems that have allowed for increased processing at a lower cost. That is in part attributable to reductions in necessary resources, changeover costs, and cleaning-validation requirements.
Large-scale, prepacked chromatography columns have recently become available for clinical and commercial manufacturing, and they represent a growing trend in the industry. Prepacked large-scale columns could meet the needs of manufacturing with single-use technologies and may have many benefits. First, quality groups may consider such columns as consumables, eliminating the need for equipment capital and qualification, asset traceability, calibration, and routine maintenance. Second, companies also see reductions in column-packing resources and associated consumables. Additional benefits may come in unexpected areas such as reduced requirements for storage space, reduced component weight for ease of handling, and improved process consistency.
When a new technology reaches the market, potential users should perform comprehensive product assessments and studies to ensure that their product characteristics and qualities are not compromised by adding it to their processes. To better understand the capabilities and limitations of prepacked chromatography columns before their implementation into a manufacturing environment, my company completed a full needs assessment to determine facility and process fit. In addition, a thorough study compared the performance of prepacked disposable columns with that of existing stainless steel column platforms at pilot scale. That study compared packed-bed integrity, column performance, and product impact. Here are some parameters that required investigation:
- Preuse, in-process, and postuse qualification results compared with vendor-provided data
- Residence time distribution (RTD) analysis of chromatograms
- Overlays of like-for-like chromatograms from each platform
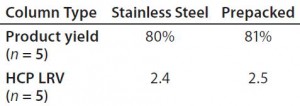
- In-process performance data, including product yield and impurity clearance
- Pressure/flow data for each packed bed.
User Requirements and Special Considerations
Prepacked columns come ready for use in manufacturing from a number of vendors. With some vendors, packed-bed height, resin type, and column diameter are limited. Several factors must be considered when a company evaluates prepacked columns for a preclinical, clinical, or commercial environment. Ideally the columns should be constructed with acceptable materials for use such as USP class VI polymers, they should be manufactured in a controlled environment, and they should provide appropriate connections to enable seamless integration into a biopharmaceutical manufacturing process. All packing procedures in producing these columns should provide consistent results. In addition, vendor-generated quality data can reduce requirements for onsite characterization by users: e.g., qualification data, endotoxin and bioburden results, packed media lot information, and a certificate of quality. It’s also important to minimize packed-column lead times because chromatography resins typically have a lead time of their own.
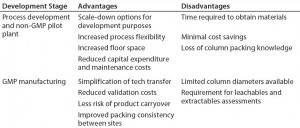
Table 1: Potential advantages and disadvantages of prepacked columns in development and manufacturing
When considering implementation of prepacked columns into a given process or site, companies should look at all potential advantages and disadvantages (Table 1). Furthermore, each company and situation is different. Development groups should perform assessments to understand the cost to implement, maximum number of expected cycles, company-specific validation requirements, product-changeover strategies, and plant downtime.
Evaluation
For this study, a prepacked 20-cm diameter OPUS column from the Repligen Corporation came axially packed to a target bed height of 10 cm with a methacrylate-based protein A resin. Shire chose Repligen as the primary prepacked-column vendor for several reasons: The company could pack it with any resin type, scale up to a 60-cm diameter with standard column dimensions, and had no set bed heights. A stainless steel 20-cm column also was axially packed with the same resin type and compression factor to a target bed height of 10 cm.
After shipment and upon receipt of the prepacked column, a standard spike qualification test was performed and its results compared with vendor qualification data found in the certificate of testing. The column initially was qualified using the same buffer matrix, flow rate, and percent injection as performed by the vendor in a test performed to ensure that shipment and handling of the column did not compromise its integrity. Results from this prequalification were slightly different from the vendor’s qualification data (Table 2): Values after shipment were greater for plates/meter and comparable for asymmetry. Those discrepancies can be attributed to system differences such as hold-up volume or dissimilarities in software used for analysis. Continued media settling during and after shipment also could have attributed to the increase in plate count.
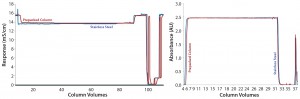
To compare in-process characteristics during product purification, the development team alternated each column for 10 capture cycles during a perfusion process. This approach generated five cycles (two harvests per capture) for each column for comparing yield and impurity clearance data. It also provided the team with an opportunity to compare process chromatograms for each platform in terms of RTD and direct overlay.
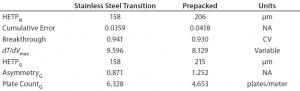
Results from in-process testing showed that both columns were comparable for overall product yield and impurity clearance (Table 3). Overlays of absorbency and conductivity traces showed comparable results for both column platforms (Figure 1). Transitional analysis (TA) of the conductivity profiles using RTD showed that the stainless steel column platform had slightly better mass transfer and higher separation efficiency than the prepacked columns, as shown in the lower heights equivalent to a theoretical plate (HETP), both Gaussian and not, as well as cumulative error values (Table 4). However, those differences can be attributed to variability in the packed bed, not necessarily the column hardware or platform itself.
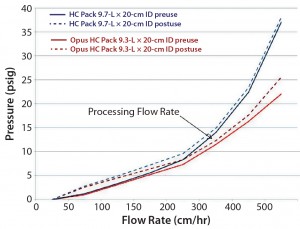
Prepacked and stainless steel columns both contained the same resin; however, differences in packing methods can affect their column pressure profiles at the same flowrate. The team completed pressure/flow profiling for both columns before use and after five runs. Column back pressures were comparable up to 300 cm/h before and after use for both formats (Figure 2). Slightly higher pressure was observed for the prepacked column at higher flow rates. Format differences such as screen size (12 µm for prepacked, 20 µm for stainless steel) and tubing were evaluated and ruled out as contributing factors. The team finally considered potential differences in slurry determination methods and/or compression factors as the more likely contributors in this case.
An Undeniable Alternative
Results of the testing described above showed only minor differences in RTD and qualification data (standard spike qualification methods) between a stainless steel platform and prepacked columns. Qualification information provided by the column manufacturer fell within acceptable ranges. And when Shire requalified the prepacked column, results showed that integrity wasn’t compromised during shipping. In addition, process characterization of product purified on each column type demonstrated comparable yields and impurity clearance, indicating no compromise of product and impurity separation.
To confirm bed integrity for each platform, column qualification performed before use, during processing, and after several runs produced consistent results, with no statistical difference for HETP and asymmetry. In addition, RTD data indicated little variability and good packed-bed integrity from cycles 1–5. Information from this study has provided justification that prepacked columns can be used in place of stainless steel columns for biopharmaceutical purification. Comparison of a prepacked column with a traditionally packed stainless steel column demonstrated acceptable overall results for all parameters evaluated.
Prepacked columns should be considered as an alternative technology for many such circumstances. Specifically, their implementation could provide an advantage in transferring biomanufacturing processes to contract manufacturing organizations. It also offers benefits for small companies without large capital and in clinical processes requiring only a few cycles to run. Such circumstances would see the greatest cost savings and benefits of large-scale prepacked columns. The demand for this type of technology is undeniable, and it is likely to continue spreading through the biopharmaceutical industry.
Further Reading
Are Pre-Packed Disposable Chromatography Columns the Future for Biomanufacturers? Chromatogr. Today 20 June 2014; www.chromatographytoday.com/news/hplc-uhplc/31/breaking_news/are_pre-packed_ disposable_chromatography_columnsthe_ future_for_biomanufacturers/30466.
Langer E. Innovation in Pre-Packed Disposable Chromatography Columns. BioPharm Int. May 2014; www.biopharminternational.com/innovation-pre-packed-disposable-chromatography-columns.
Corresponding author Shaun Grier is associate director, and Saani Yakubu is a senior development specialist in downstream pilot-plant operations at Shire Pharmaceuticals, 300 Shire Way, Lexington, MA 02421; 1-617-349-0200; www.shire.com.