This webcast features: Fletcher Malcom, Director of Product Management, Repligen With an innovative side port that allows for easy resin unpacking, OPUS® 45R and OPUS® 60R Columns provide the ultimate flexibility in pre-packed column technology. Without compromising chromatographic performance or cleanability, the new feature helps mitigate the risk of implementing pre-packed columns in CGMP settings, and allows for re-use of the unpacked resin in other columns. This webinar covers test results on chromatographic performance, bioburden testing, shipping tests and simple…
Webinars
Enabling Custom Solutions for Downstream Processing for Future Therapies: AAV Case Study
This webcast features: Orjana Terova, Purification Product Manager, Thermo Fisher Scientific There is a need in the industry for a standard purification strategy for the manufacture of novel molecules including viral vectors and oligomeric therapies. Working with customers, Thermo Fisher Scientific has developed innovative purification resins through the POROS™ custom resin program and CaptureSelect™ ligand technology. This combination of ligand and resin development has allowed for development of platform purification processes that increase productivity and improve product yield for gene therapies.…
Challenges of Scale Up and Development of Insect Cell Culture and BEVS
This webcast features: Sharyn Farnsworth, Associate Principal Scientist, FUJIFILM Diosynth Biotechnologies A strategy for scale up and implementation of Insect Cell Culture (ICC) should be assessed at the earliest stages of process development as there are a different set of challenges in the culture of ICC and BEVS versus a CHO culture and a limited number of vendors experienced enough to guide you through the adventure of manufacturing ICC. Each system has a unique problem set ranging from which cell…
The Total Cost of Buffer Preparation and Handling
This webcast features: Eric Langer, Managing Partner, BioPlan Associates, Inc. Most bioprocessing facilities consider in-house preparation of buffers and bulk liquids to be a core bioprocessing task. However, some companies are now outsourcing liquid manufacturing, by purchasing ready-to-use materials from vendors or hiring CMOs to prepare these. Buffers and bioprocessing liquids are one area of upstream production operations that is seeing an increase in outsourced operations. Bulk liquids can be a bottleneck in downstream processing, with buffer volumes often required…
Single-use XCell ATF Systems for Continuous Processing: 100% Cell Retention, 80% Faster Set-Up
This webcast features: Christine Gebski, Vice President, Product Management and Field Applications, Repligen In today’s flexible manufacturing environments and to improve manufacturing cost of goods, many companies have implemented single-use equipment. To meet these changing customer requirements, the XCell™ ATF 2 and ATF 6 are now available in single-use formats. The XCell ATF Single-use systems deliver the same cell culture performance as the stainless steel systems but with 8x faster set-up time and a simplified autoclave-free implementation workflow. These elements…
Taking Medium and Feed Development Beyond Maximizing Protein Titer to Optimizing Glycan Distribution and Simplifying Process Scale-Up
This webcast features: Serena Fries Smith, Process Science Manager, Thermo Fisher Scientific In the early 2000s when many processes were struggling to achieve 1 g/L, maximizing titers was the industry’s biggest challenge and was essential to having favorable cost of goods and an economically viable product. Over the last 1–15 years, the industry has made significant advances in media and feeds. Due to these advancements, today a standard fed-batch process can typically achieve 3 g/L and some processes are achieving…
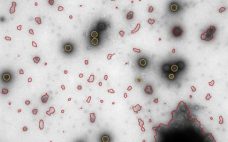
Reveal Information That Gives Insights: New Approaches to Sub-Visible Particle Characterization
This webcast features: Josefina Nilsson, Head of EM Services, and, Gustaf Kylberg, Product Manager – MiniTEM, Vironova Sub-visible particle characterization is essential when comparing sample quality after different purification steps or for the understanding of product stability. Analysis performed with MiniTEM provides both morphology and accurate quantitative data that can help speed up process and formulation development. In this webinar you will learn: How MiniTEM automatically images, detects and classifies a large number of particles resulting in statistically significant and…
Improving Single-Use Bioreactor Design and Process Development: New Research Towards Intensifying Seed-Train and Scale-Up Methods Using 5:1 Turn-Down
This webcast features: Surendra Balekai, Senior Global Product Manager, Thermo Fisher Scientific Bioproduction Operating bioreactor vessels at low working volumes (low turn-down ratio) is often desirable but brings about challenges in regard to mixing, mass transfer, and process control. Research done toward optimizing cell culture has provided methods to improve performance and control when operating under these conditions. Implementing bottom heat exchange, making changes to impeller angle and height, taking advantage of the unique Thermo Fisher drilled-hole sparge design, and…
Top 5 Considerations When Looking to Outsource to a Biomanufacturing Partner
This webcast features: Paul Jorjorian, Director, Global Technology Transfer, Patheon Biopharmaceutical companies are facing increasing constraints when developing and manufacturing their large molecule drugs. Development labs and manufacturing suites have limited capacity making it nearly impossible to right size internally for uncertain forecasts. As a result, selection of the right biomanufacturing partner has never been more important – for small and mid-size biopharma companies alike. There are an ever-increasing number of options and many criteria to consider during the vendor…
Practical Aspects of Implementing Continuous Bioprocessing
This webcast features: Dr. Marc Bisschops, Principal Scientist, and Dr. Mark Schofield, Principal Engineer, R&D, Pall Life Sciences The next evolution in the biopharmaceutical industry is the widespread adoption of integrated continuous bioprocessing for biologics manufacturing. The key to its success, however, is the availability of novel upstream and downstream technologies that will not only reduce facility footprint, capital expenses, and product cost of goods, but will also increase process productivity, flexibility, and further facilitate the utilization of single-use and/or…