This webcast features: David Howes, ILC Dover Learn about modern single-use powder containment best practices and how to reduce cross contamination and airborne particulates.  You will gain insight on how you can increase safety and speed when handling powders. In the study, we will discuss the ease of fill along with dispensing times and product loss. ILC Dover’s EZBiopac® shows a 71% decrease in filling times when measured against an industry standard 2D bag. Due to the non-static film we…
Manufacturing
Proper Task Performance in GMP BioManufacturing: Human Error Reductions
This webcast features: Tony Fultz, Director of Upstream Manufacturing at Fujifilm Diosynth Biotechnologies. Human error reduction should be a top priority of every organization. Our company, clients, and most importantly, our patients are heavily dependent on right first time execution. Human error is, and will likely always be, the highest contributor to deviations and batch failures. Fujifilm Diosynth Biotechnology (FDB) is aggressively seeking to reduce human error. Using his experience in the nuclear industry, Tony Fultz shares a method implemented…
Routinely Identify Low-Level Attributes – Fast and Accurately
This webcast features: Sibylle Heidelberger, Technical Marketing Manager for Biologics, SCIEX. Biotherapeutic peptide mapping LC-MS acquisition methods can make it difficult to routinely identify low-level modifications, such as deamidation and oxidation. This webinar discusses how the use of generic SWATH® Acquisition method on the benchtop X500B QTOF System and BioPharmaView™ Software allows you to confirm the identity of low-level attributes with fast and accurate batch analysis and processing. She also presents how the use of this unbiased data-independent SWATH® Acquisition…
Collaborate to Innovate: A Webinar Series
As a senior leader in pharmaceutical development and manufacturing, you know that keeping up-to-date on industry trends is critical to maintaining competitive edge. But do you know how to actively drive these trends and apply them to your manufacturing processes? Are you collaborating with the right stakeholders in order to expand your capabilities and bring your innovation strategy to life? In this webinar series, three subject matter experts will share their perspective on the future of the industry and provide…
Accelerating the Biotech Value Chain Through the Implementation of High-Throughput Technologies
This webcast features: Fredrik Nilsson, Director of Downstream Development, and Jesper Worm, PhD, Scientist, Department of Analytical Development, at CMC Biologics. In this webinar, the speakers discuss and analyze the use of high-throughput technologies to optimize timelines and deliver value within the upstream and downstream development processes, using Octet HTX platform technology. Over the past year, the experts at CMC Biologics have used this technology to deliver a significant number of titer data points, and residual protein data measurements. This…
Advanced Materials for Single-Use Biomanufacturing Systems
This webcast features: Michael W. Johnson, Business Development Engineering Manager, Life Sciences, Entegris Utilizing advanced fluoropolymer materials in single-use systems has distinct advantages in regards to extractables, chemical compatibility and cold temperature performance. This webcast examines data obtained from pilot scale testing of a new fluoropolymer single-use bag system for the freezing, storage and shipping of formulated bulk biopharmaceutical product.  You will learn: What material has the widest temperature profile from +200 to -200°C Which film material (LDPE, Polyolefin, EVAM,…
Improving Protein Folding Control and Scalability Using imPULSE Mixing Technology
This webcast features: Anthony Hawrylechko, Director of Microbial Bioprocess, Cytovance Protein folding by dilution is a common approach used in the manufacturing of biologics derived from microbial expression systems. This typically involves the solubilization of a washed inclusion body preparation containing the concentrated product polypeptide with a strong chaotrope or detergent solution. The denatured, inactive product solution is then diluted into a combination pH and red/ox buffer solution. Within this environment, the molecular diffusion rates of chaotrope, buffer components, and…
The Total Cost of Buffer Preparation and Handling
This webcast features: Eric Langer, Managing Partner, BioPlan Associates, Inc. Most bioprocessing facilities consider in-house preparation of buffers and bulk liquids to be a core bioprocessing task. However, some companies are now outsourcing liquid manufacturing, by purchasing ready-to-use materials from vendors or hiring CMOs to prepare these. Buffers and bioprocessing liquids are one area of upstream production operations that is seeing an increase in outsourced operations. Bulk liquids can be a bottleneck in downstream processing, with buffer volumes often required…
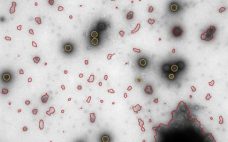
Reveal Information That Gives Insights: New Approaches to Sub-Visible Particle Characterization
This webcast features: Josefina Nilsson, Head of EM Services, and, Gustaf Kylberg, Product Manager – MiniTEM, Vironova Sub-visible particle characterization is essential when comparing sample quality after different purification steps or for the understanding of product stability. Analysis performed with MiniTEM provides both morphology and accurate quantitative data that can help speed up process and formulation development. In this webinar you will learn: How MiniTEM automatically images, detects and classifies a large number of particles resulting in statistically significant and…
Top 5 Considerations When Looking to Outsource to a Biomanufacturing Partner
This webcast features: Paul Jorjorian, Director, Global Technology Transfer, Patheon Biopharmaceutical companies are facing increasing constraints when developing and manufacturing their large molecule drugs. Development labs and manufacturing suites have limited capacity making it nearly impossible to right size internally for uncertain forecasts. As a result, selection of the right biomanufacturing partner has never been more important – for small and mid-size biopharma companies alike. There are an ever-increasing number of options and many criteria to consider during the vendor…