The International Society for Cellular Therapy (ISCT) will host its 21st Annual Meeting at Caesars Palace Hotel and Convention Center, Las Vegas, NV, 27–30 May 2015. More than 1,200 industry and regulatory professionals, clinicians, scientists, and laboratory professionals are expected to attend. The program covers six plenary sessions, six workshops, three technical sessions, and more than 20 total track sessions covering such topics as advances in cell therapy research, commercialization strategies, quality and operations, and regulatory issues. BPI spoke with…
Manufacturing
Special Report on Product Stability Testing: Developing Methods for New Biologics and Emerging Markets
Stability testing is a vital part of product development and is conducted throughout a product’s life cycle (Figure 1). Stability is part of a biotherapeutic’s quality target product profile, and results help analysts understand how critical quality attributes (CQAs) of both drug substances and products are influenced under specific conditions of temperature, relative humidity (RH), light, storage, pH, and other factors. Manufacturers conduct stability tests to determine degradation pathways and establish shelf lives and storage conditions of their products, for…
Information Instead of Data: User-Friendly HMI Concept Increases Process Control Efficiency
Today, most plant operators are much more than classic process operators. In addition to operational process control, their range of tasks includes product quality assurance, optimization of resources, and maintenance of high‑throughput rates. Qualified information is necessary to reliably perform those tasks. The human–machine interface (HMI) of a process control system provides visualization. Siemens regards new HMI concepts such as advanced process graphics (APG) as the key to task‑specific or situation‑specific decision‑making (Photo 1). Graphics Monitor: the Process Window Before…
Accelerating Affordable Growth: Careful Planning Can Pave the Way for Commercial-Scale Manufacturing
As this special issue of BioProcess International goes to press, an increasing number of cell-based therapies are advancing through preclinical investigation into clinical development and on toward commercialization. Although clinical efficacy will be the primary metric for product approval, the ability to manufacture these therapeutic products consistently, reliably, and cost-effectively will continue to be a key driver and predictor of commercial success. Several articles presented herein describe major issues and challenges facing developers of cell-based therapies. Although some of the…
Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 1: Achieving Manufacturability and Managing Cost of Goods
Much mystique and mystery surround the emerging industries of cell therapy and regenerative medicine. As companies progress toward commercial manufacture with potential game changers (e.g., cures for cancer and diabetes) the industry could be on the verge of significant breakthroughs. However, with no real successes to date, the question is raised: What core attributes are required to achieve commercial success? The tale of Dendreon’s struggles highlights how difficult it can be to commercialize even an approved cell therapy product. Since…
Foundation Elements for Cell Therapy Smart Scaling
Cell therapy is the injection of cellular material into patients. The injected cell-therapy product (CTP) usually consists of intact living cells. In recent years, cell therapies have evolved and matured, moving from academia to industry. That maturation is reflected in the number of open clinical trials that include the term cell therapy in their descriptions: To date, there are more than 8,700 open trials listed on the US National Institutes of Health’s online database (clinicaltrials.gov), most of which are in…
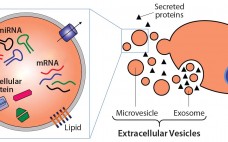
Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (1, 2). Sometimes called exosomes, they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential. In light of growing efforts in using EVs for research and therapy,…
Maximizing PMBC Recovery and Viability: A Method to Optimize and Streamline Peripheral Blood Mononuclear Cell Isolation, Cryopreservation, and Thawing
The quality of peripheral blood mononuclear cells (PBMCs) isolated from whole blood has a significant impact on their subsequent analysis. Maximizing recovery, viability, and functionality of isolated PBMCs is essential to the reliability and consistency of downstream applications, particularly within cell therapy manufacturing. The standard method for purification of PBMCs is density-gradient centrifugation. It requires precise layering of whole blood over a density medium (e.g., Ficoll polysaccharide reagent from GE Healthcare), with careful pipetting of the floating cell layer after…
The Potential Application of Real‑Time Release Testing for the Biomanufacture of Autologous Cell‑Based Immunotherapies
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (1) for a range of clinical indications and unmet therapeutic needs — particularly oncology. The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies…
Are You Ready for a Tech Transfer? Part 1: Challenges and Critical Factors for Success in Cell Therapy Development
Cell therapies offer enormous promise for treatment of a range of conditions by replacing damaged tissue or leveraging the body’s own resources to heal itself. Not surprisingly, the cell therapy industry is growing rapidly and is poised to have a major impact on healthcare and disease treatment. The Alliance for Regenerative Medicine (ARM) has reported on the robust state of the industry, noting that revenue from cell-derived products grew from US$460 million in 2010 to $1.3 billion in 2013 (1).…