Many biotherapeutic proteins are naturally subject to aggregation. The clinical consequences of protein aggregation can be dramatic, not only affecting bioavailability and pharmacokinetics, but in extreme cases dramatically altering pharmacodynamics as well. Of equal or perhaps more importance is that aggregation is a principal source of unwanted immunogenicity in biotherapeutics. Aggregation-induced neutralizing antibodies and/or anaphylactic reactions are serious and growing US and European regulatory concerns. So they will have significant and growing influence on the future development and regulatory approval…
Manufacturing
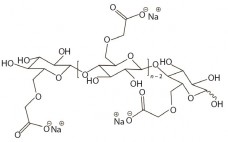
Factors Affecting Sterile Filtration of Sodium-Carboxymethylcellulose–Based Solutions
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (1–3), serves as a suspension aid (4), and stabilizes emulsions (5). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (6, 7). CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree…
Insulin in Demand: Engineering a Facility to Serve Local and International Markets
Discovered in 1922 at the University of Toronto (by Frederick Banting and John James Rickard Macleod), insulin has been produced from animal extract, primarily cattle and pigs. Following Frederick Sanger’s work on insulin sequence in 1951, Dorothy Hogkin published in 1969 the three-dimensional structure of insulin. This breakthrough led to many developments and applications of recombinant DNA for the production of insulin, human first and then analogs. The significant increase in the diabetic population — especially in BRICS countries (Brazil,…
Responding to an FDA Form 483: A Five-Step Approach
When the US Food and Drug Administration (FDA) inspects your company’s biomanufacturing facility, investigators use the FDA Form 483 to record observations and findings (1). Such inspections typically review all good manufacturing practices and good laboratory practices (GxP) quality systems documents. If those investigators find compliance issues, they deliver a summary of their observations and findings using a Form 483, a copy of which will be provided to your company at the end of the inspection visit. How to Respond…
Automated Purification of Native and Recombinant Proteins Using Multidimensional Chromatography
In traditional sequential chromatography, columns are run as separate entities. The process requires significant hands-on time and constant manual intervention. By contrast, automated chromatography technology provides the same results more efficiently and reliably and frees researchers to focus on other tasks, thereby shortening protein purification times from days to hours. For drug discovery, purifying protein samples is required to generate enough materials for research experiments. But the process is complex and time consuming. It involves repeated single-column purifications, careful analysis,…
Development, Qualification, and Application of a Bioreactor Scale-Down Process: Modeling Large-Scale Microcarrier Perfusion Cell Culture
Qualified scale-down models of large-scale cell culture processes are essential to conducting studies for applications such as investigating manufacturing deviations, enhancing process understanding, and improving process robustness. For example, scale-down models can be used for raw material investigations as well as evaluation and qualification of new good manufacturing practice (GMP) cell banks for manufacturing implementation. Process characterization studies are performed also with qualified scale-down models to improve process consistency (1, 2). Often it is impractical to conduct investigational studies at…
Accelerating Biologic and Biosimilar Drug Development: Ready-to-Use, Cell-Based Assays for Potency and Lot-Release Testing
With the drug industry’s expanding emphasis on biologics, the need for robust cell-based assays has grown at all stages of development. Requirements for efficacy, quality, and potency testing often demand a complex set of bioassays and/or cell-based assays for new therapeutics or biosimilars. Developers of the latter have found this need for cell-based assays to be particularly challenging. Commercially available, ready-to-use cell-based assays provide a robust functional response from specific therapeutic targets. They can significantly shorten assay development time while…
Ask the Expert: FOLDTEC Refolding of Biopharmaceuticals A Case Study of Recombinant Thrombin
with Dr. Andreas Anton and Dr. Sebastian Schuck Poorly soluble substances form aggregated inclusion bodies (IBs) in microbial cells containing incorrectly and/or incompletely folded target proteins. Wacker Biotech, a full-service contract manufacturer of biopharmaceuticals based on microbial systems, has introduced FOLDTEC refolding technology for bioengineered therapeutic proteins. The proprietary platform uses specifically developed and optimized bacterial strains and a patented, antibiotic-free expression system. In a BPI webinar on 9 November 2015, Wacker’s director of bioprocess development (Andreas Anton) and head…
Elucidation: Strict, but Flexible, Industrial Automation for Biopharmaceutical Manufacturing
Biopharmaceuticals are the fastest growing sector of the pharmaceutical industry, making up about 20% of the market, with annual growth rates of about 8% (double that of more traditional pharmaceutical sectors). To increase capacity and uphold stringent quality and regulatory demands, manufacturers often reassess their operational and technology strategies while focusing on rising manufacturing costs and the pressure of delivering cost-effective new drug products. Bioproduction can range from small batches to low-cost, high-volume campaigns. Few manufacturers have the required in-house…
Special Report: Toward Sustainable Engineering Practices in Biologics Manufacturing
Introduction by William P. Flanagan Biopharmaceutical development and manufacturing demand scalable processes that can be quickly developed, easily implemented, and smoothly transferred to production. Disposable, ready-to-use technologies play a crucial role in providing flexibility to support agile biomanufacturing operations. Single-use systems provide process efficiencies by removing steps such as cleaning and cleaning validation, thus allowing for faster change-over between manufacturing runs. The biopharmaceutical industry is increasingly adopting single-use approaches, and the global market for such bioprocessing tools is expected to…