Analytics pervade the entire biopharmaceutical development process — from protein characterization through biomanufacturing process optimization to final-product formulation and clinical testing. Every technical article in BPI requires data to back up the statements made, whether the topic is upstream/production, downstream processing, product development, or otherwise focused. And never mind publishing: Even more detailed documentation is required for regulatory submissions. If a company can’t back up the choices made and results obtained in development, manufacturing, and testing of its biopharmaceutical product,…
Manufacturing
eBook: The Future of Monoclonal Antibody Manufacturing — Incremental Improvement or Industrial Revolution?
Monoclonal antibody manufacturing is at a crossroads. Biomanufacturers could continue exploring new technologies and fine-tuning proven systems such as mammalian cell expression systems in stirred-tank bioreactor fed-batch cultures. But some experts say an opportunity is arising to turn the industry on its head by taking lessons from other branches of bioprocessing, such as the industrial enzyme sector. Drug makers are criticized often these days for the high prices of their products. The lay media, governments, payers, and patients themselves all…
eBook: The Commercial Expression Systems Market — What Has Changed in the Past Decade
A decade ago, BioPlan Associates prepared the findings of its 2008 directory of expression system technologies that were being promoted or considered likely to be suitable for commercial licensing for biopharmaceutical manufacturing (1). Due in part to the relatively slow advances in this critical area of bioprocessing, this study remains perhaps the only directory of biopharmaceutical-relevant expression systems available for licensing. Here I discuss aspects of related bioprocessing technologies that have and have not changed in the past decade. Expression…
Facilities of the Future: Intelligent Design and Control Enable Quality, Efficiency, and Good Citizenship
Today’s biomanufacturers need to be able to add capacity and capability quickly; provide increased supply service to customers on demand; and streamline the flows of personnel, traffic, utilities, and materials throughout bioprocess facilities. And companies need to be flexible enough to subtract capacity and retool quickly to produce new or different products. Many future facilities will be automated to some extent and use robotics in manufacturing. With personalized medicine on the rise, bioprocessors can benefit from colocation with academic research…
Flexibility, Automation, and Leadership: Drug-Sponsor Perspectives on Modern Biomanufacturing Facility Design
The title of this featured report — Smart(er) Facilities — came about in conversations with our KNect365 colleagues as they worked to plan this year’s BPI West conference, which took place 19–22 March 2018 in San Francisco, CA. For decades, biopharmaceutical facilities have incorporated cutting-edge designs for supporting processes, products, and human development. Each year, design innovations are rewarded for creating workspaces that facilitate both worker comfort and essential movement of promising drug candidates toward commercialization. In that context, biomanufacturing…
Partnerships for Progress: Supplier Perspectives on Facilities of the Future
Biomanufacturers are constantly tasked with making their products ever more efficiently with ever increasing quality. As major advancements come in biomanufacturing technology, companies find themselves in need of “smarter,” more flexible facilities than ever before. Recently, I asked several industry leaders for their thoughts on some criteria for smarter designs, including why there is such a demand: Cristina Amorim (vice president of facilities, EHS, and sustainability in the life sciences group of Thermo Fisher Scientific) Scott Battist (vice president, general…
Cost Analysis of Cell Therapy Manufacture: Autologous Cell Therapies, Part 2
In part 2, we continue to analyze manufacturing costs of an autologous cell therapy. A typical process involves the expansion and activation of cells derived from a single patient, which is currently very labor-intensive. To date, there is little published information on overall production costs (1). In part 1, we used a software modeling platform to identify opportunities for potential cost savings. We developed a baseline model of a cell therapy manufacturing process using the production of autologous dendritic cells…
Data Science, Modeling, and Advanced PAT Tools Enable Continuous Culture
Bioprocesses traditionally use (fed-)batch cell culture processes for production of recombinant proteins and therapeutics. In batch bioprocessing, material flow is discrete, with a hold step between two unit operations, and product is harvested only once for each unit operation. Batch processes have been studied extensively and optimized through numerous advancements in experimental design (1, 2), monitoring (3–5), measurement techniques (6–9), and control strategies (10–12). However, such processes require large facility footprints for equipment (13) as well as sterilization, load, and…
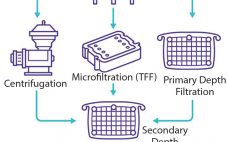
Filter-Based Clarification of Viral Vaccines and Vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Ensuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 ÎĽm
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.…