On Thursday 6 September 2018 at the annual BioProcess International Conference in Boston, the first ‚ÄúTechnology Round Robin Featuring Six Innovative Bioprocess Technologies‚ÄĚ was presented in an interactive session with attendees as active participants, asking questions and engaging in conversation with the six featured entrepreneurs. Detailed below, this session was a culmination of several steps in an overall strategy for some of the companies participating. To fully appreciate the launch of new technologies into the bioprocess arena, you first must…
Manufacturing
Rolling with the ‚ÄėTides: Elucidating the Role of Peptides and Oligonucleotides in the Biopharmaceutical Industry
In earlier issues of BPI we published a few ‚ÄúElucidation‚ÄĚ closers that we called ‚ÄúDefining Moments.‚ÄĚ Since then, we have tried to distinguish key confusable terms from one another. Those presented (and sometimes ‚Äúelucidated‚ÄĚ) have been analytical and bioanalytical, spectroscopy and spectrometry, and biosimilars and biobetters. They are just a few of the many confusable terms in the biopharmaceutical industry. For example, when someone says ‚Äúdrug delivery,‚ÄĚ a formulator will think of a syringe or transdermal patch, but a logistics…
The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics
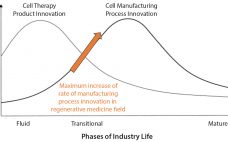
Most commercial biopharmaceuticals originated from academic research laboratories and start-up development laboratories. Despite such products having differences in modalities and targeted disease indications, and whether their target patient populations are relatively small or approaching blockbuster status, at a key point in development, biopharmaceutical production must scale up from laboratory to commercial production. That movement from research to development and then to manufacturing forces attention on economics and speed to market, and it drives innovative approaches to producing biopharmaceutical cell compositions…
Development and Application of a Simple and One-Point Multiparameter Technique: Monitoring Commercial-Scale Chromatography Process Performance
In commercial-scale biopharmaceutical manufacturing, downstream chromatography steps are still a bottleneck and contribute to significant operational costs (1, 2). Some of those costs are inherent (e.g., resins, large buffer quantities, and cleaning) whereas others are avoidable (e.g., product loss due to rejected lots or deviations that result in production downtime). Maintaining efficient and robust chromatography process performance is therefore critical for minimizing operating costs. To do so, we introduce a simple and one-point multiparameter technique (SOP-MPT) for monitoring chromatographic process…
Multitiered Automation for Improved Efficiency of Bioprocess Analytics
The first biopharmaceutical, human insulin, was approved for use in 1982 (1). The biopharmaceutical market continues to exhibit healthy growth now, with the number of yearly patent applications increasing by 25% annually since 1995 (2). The total pharmaceutical R&D pipeline has more than doubled since the beginning of the century (Figure 1), much of that attributable to the biologics industry segment. As this industry has matured, new platform methods have emerged, and competition has increased. Consequently, the pressures of speed,…
eBook: Using Modern In Situ Analytics and PAT for Automated Feedback Control of Critical Process Parameters
Simply put, the best way to control a critical process parameter (CPP) is to measure that specific parameter, integrate the live signal into your control system, and apply a smart feedback algorithm for an automated control loop. The challenge in doing this for bioprocesses has been due, in part, to the complex, highly dynamic, and variable nature of the process along with the lack of robust, scalable, and multiformat (single-use or multiuse) technologies that can monitor in real time such…
Partnerships in Immunotherapy: Working Together to Take Cancer Treatment to the Next Level
Biopharmaceuticals are a particularly complex expression of medicine ‚ÄĒ and immunotherapies perhaps even more so. As treatments, these products themselves often also need ‚Äúpartners‚ÄĚ of a kind: e.g., radiation/radiotherapies, traditional MAbs, and chemotherapies. Just as this field of endeavor requires the input and expertise of many different disciplines ‚ÄĒ from medical researchers to process engineers, clinicians to business leaders, and market experts to policy makers ‚ÄĒ this discussion of the topic of partnerships in immunotherapy brings together different experts in…
Development of a Standardized Extractables Approach for Single-Use Components: General Considerations and Practical Aspects — A Manufacturer’s Perspective
The subject of extractables for single-use bioprocess contact materials has been a subject of heated debate since roughly the summer of 2012, when the first ISPE paper was published issuing a call to action to develop a standardized extractables protocol for the industry (1). As a supplier that pioneered the science of extractables (2‚Äí11) and has published extractables data for our products for over 20 years, Sartorius Stedim Biotech (SSB) took the opportunity to look back, take stock, rationalize, and…
A Novel 3D Culture System for High-Throughput Hepatoxicity Screening
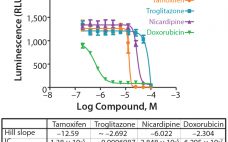
Cells grown as three-dimensional (3D) spheroids are thought to more closely mimic in vivo physiology in terms of morphology, structural complexity, and phenotype. Being more physiologically relevant, 3D cultures can be highly predictive for compound profiling and evaluating cytotoxicity, a critical step in evaluating chemotherapeutic drug candidates. Unfortunately, evaluation of drug cytotoxicity traditionally has relied on the use of two-dimensional (2D) cell culture monolayers. When grown in monolayers, cells are not exposed to soluble gradients, are forced into an apical-basal…
Biological Stealth Bombers: Potency, Regulatory, and Bioprocessing Concerns of Antibody‚ÄďDrug Conjugates
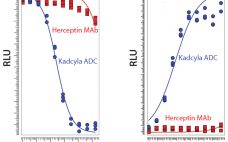
Seven years ago, the US Food and Drug Administration (FDA) approved the first product in a new class of biologics: antibody‚Äďdrug conjugates (ADCs). The idea for these products already had been hatched a decade earlier when the promising field of antibody research ‚ÄĒ touting such molecules as ‚Äúmagic bullets‚ÄĚ ‚ÄĒ had faltered, specifically against oncology-related indications. The early crop of anticancer monoclonal antibodies (MAbs) proved to have only limited efficacy, and interest in developing antibodies as therapeutic agents against cancer…