The Sartorius upstream portfolio addresses key strategic challenges facing the biopharmaceutical industry: Increased speed to clinic/market and lowered capital costs, with improved process control. Fully scalable, proven process solutions for cell line, media, and process development through commercial manufacturing accelerate upstream development and simplify manufacturing. Novel high-throughput development tools for intensified processes incorporate the latest in process analytics, multivariate data analysis (MVDA), and design of experiments (DoE) software tools. These tools are designed to compress development timelines and to scale…
Manufacturing
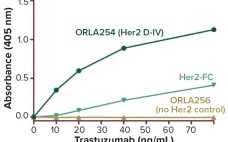
Building Toward Antibody‚ÄďDrug Conjugate Success
‚ÄúWe are witnessing one of the most significant paradigm changes in oncology drug development, with some new types of immunooncology compounds inducing unprecedented increases in survival in certain solid and liquid tumor indications.‚ÄĚ ‚ÄĒJagath Reddy Junutula (Cellerant Therapeutics) and Hans-Peter Gerber (Pfizer) (1) An antibody‚Äďdrug conjugate (ADC) for cancer treatment needs four things to succeed clinically: It needs to target the right antigen using the right antibody, with the most powerful cytotoxin attached by best linker option. These features combine…
Antibody‚ÄďDrug Conjugate News: From BioProcess Insider
The following news items have appeared on the BioProcess Insider site over the past year. Together they indicate the direction in which the ADC sector is moving. BEYOND ADCETRIS ‚ÄĒ SEATTLE GENETICS AIMS FOR BIG BIOPHARMA STATUS 30 April 2018: Seattle Genetics is building toward a bigger future. For the first quarter 2018, the company‚Äôs total revenues grew to US$141 million (‚ā¨116 million) compared with $109 million in the same period last year. This was attributed to a 36% increase…
Analytical Tools to Improve Decision-Making During Product Development
Speed to clinic testing ‚ÄĒ and then speed to market ‚ÄĒ are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial ‚Äúdesign of experiment‚ÄĚ (DoE) analysis…
Biologic Labels and Induced Patent Infringement: A Perspective on Evolving US Law
The mechanism for proving patent infringement is changing for developers of both branded and follow-on biologics (either biosimilar or interchangeable). Here we examine how drug labeling can establish infringement, thus affecting follow-on manufacturers accused of inducing others to infringe patents on methods of treating medical conditions. Because precedent is paramount in the US legal system, judges look to small-molecule case history to help them understand alleged infringement by follow-on biologics. The classic approach to induced infringement of generic small-molecule drugs…
The Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production –
Part 2: Regulatory Overview
The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plasticequipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA‚Äôs core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and…
Integrity Redefined: Consistent Robustness and Integrity Testing Lead to Enhanced Process Integrity and Patient Safety
With the increasing adoption of single-use systems (SUS) in critical stages of biopharmaceutical manufacturing, any lack of system integrity can significantly affect drug product quality and patient safety, as well as incur additional costs due to product loss and disrupted production cycle. This article from Sartorius Stedim Biotech, describes how determining the correlation between liquid leakage and microbial ingress can be used to define MALLs (Maximum Allowable Leakage Limits) of SUS for different process steps. The article also details the…
A Harmonized Approach to Data Integrity
Data integrity is achievable when data collection is complete, consistent, and accurate (1). Failure to maintain data integrity compromises a company‚Äôs ability to demonstrate the safety and efficacy of its products. Escalation of serious regulatory actions related to data integrity violations has prompted the need to assess data integrity compliance and implement systems designed to guarantee it. Comprehensive measures must be taken to ensure that data are attributable, legible, contemporaneous, original, and accurate (ALCOA) (2). Preventive measures need to be…
Opportunities for Modern Robotics in Biologics Manufacturing
It should come as no surprise to anyone familiar with biomanufacturing that current designs of bioprocess facilities as well as associated manufacturing spaces and support operations require excessive amounts of manual labor and manual interventions that lead to high labor costs and, consequently, total cost to supply. From receipt of raw materials to process execution and performance review, resolution of quality issues, and product shipping, no industry devotes a greater percentage of operating costs (or cost of goods sold, CoGS)…
Determining Control Chart Limits for Continued Process Verification with Autocorrelated Data
Control charts are used to assist in process monitoring activities. They use an estimate of central tendency (the overall mean) and variation (the standard deviation). Sample standard deviations (S) tend to underestimate process standard deviations (ŌÉ) when they are calculated using limited sample sizes of independent results (1). For this reason, the unbiasing constant c4 is used as a divisor when calculating Shewhart control-chart limits. If data used for control charting are positively autocorrelated, that tends to underestimate ŌÉ further…