The SAMPLE program — a Standard Approach to ATMP Tissue collection — is intended to help build capacity for the UK National Health System (NHS) to expand the use of next-generation cell and gene therapies for both cancer and noncancer illnesses. Now it has been given the green light by Innovate UK (IUK), the United Kingdom’s technology strategy agency. An award through the Industrial Strategy Challenge Fund is supporting the program for standardizing how cell and gene samples are collected…
Manufacturing
Immunotherapy: Taking Aim at Solid Cancers
As cell and gene therapies arrive on the market, all eyes have focused on autologous chimeric antigen receptor (CAR) T – cell therapies. At the 2019 Phacilitate Leaders World and Stem Cell Summit in Miami, FL, delegates looked at where the biopharmaceutical industry is going in the cellular immunotherapy space. Whether for off-the-shelf CAR T-cell products, personalized cancer vaccines, or modified natural killer (NK) cells derived from human induced pluripotent stem cells (iPSCs) — cell and gene therapy development is…
Supply Chain Solutions for Cell and Gene Therapy Companies
Stakeholders across the supply chain stress that quality of starting material will be key to the success of cell and gene therapies. This is a topic that has created issues in the past, is puzzling the industry presently, and is likely to cause more problems going forward. This topic was front and center at the 2019 Phacilitate Leaders World and World Stem Cell Summit in Miami FL, with presentations focusing on supply chain solutions to address these complex challenges; cell…
Automation in Cell and Gene Therapy Development
The US approvals of chimeric antigen receptor (CAR) T-Cell therapies Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel) and gene therapy Luxturna (voretigene neparvovec) in 2017 heralded a “new frontier of medicine.” But with great innovation comes great costs and criticism (such as the Kymriah’s US$475,000 price tag). Many companies argue that these one-off therapies represent good value for patients and payers compared with traditional treatments, however, no matter your perspective, the COGs picture for cell and gene therapies isn’t good and…
Manufacturing of Cell and Gene Therapies
The peak of demand for curative cell and gene therapies will be unlike that of traditional drugs and thus could cause forecasting and overcapacity issues going forward. Predicting the future is always difficult, and poor decisions can be costly and highly damaging for a company. At the 2019 Phacilitate Leaders World and Stem Cell Summit in Miami, FL, several presentations and conversations focused on the forecasting dilemma and how manufacturing needs innovation, just to name two. This eBook details these…
eBook: Allogeneic Cell Therapies Commercialization Strategies
Manufacturers of allogeneic cell therapies face development and commercialization challenges unlike those of traditional cell therapies. In a discussion with Phil Vanek of GE Healthcare, we outline several of the key challenges of processing these products and bringing them to market. Approaches for reducing development costs and lowering pricing are highlighted, and a separate analysis presents three pricing models specific for allogeneic “off the shelf” cell products.
Large-Scale Capacity Strategies: Single Use, Multiuse, or Both?
Early manufacturing facilities for large-scale production of biopharmaceuticals were, by necessity, very large. Low expression titers and blockbuster-market products such as monoclonal antibodies (MAbs) combined to require massive bioreactors — with capacities of 10,000– 20,000 L or more — and supporting infrastructure. In my early days covering the industry, I visited a few such facilities and was always awed by the huge tanks and what seemed like miles of piping. A 2010 Pharmaceutical Engineering article described a process modeling approach…
Continuous Biomanufacturing: A New Approach to Process Scale
The BioPhorum first-edition Technology Roadmap outlined a 10-year vision for therapeutic protein production in the biopharmaceutical industry (1). The roadmap describes multiple manufacturing scenarios ranging from large-scale (~20,000-L production) to small and agile, portable production facilities. It includes detailed analyses of the needs for the future in each of the following areas: Process technologies (2) Inline monitoring and real-time release (3) Automated facilities (4) Modular and mobile (5) Knowledge management (6) Supply partnership management (7). Since the 2017 publication of…
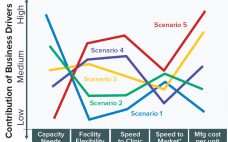
Biomanufacturing Scenarios: From the Biomanufacturing Technology Roadmap
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
A Shift of Mindset: How Single-Use Systems Influence Bioprocess Engineering and Project Execution
In past decades, the focus in bioprocess engineering was on traditional stainless steel project management, which is highly dependent on established project schedules. Many milestones, tasks, and deliverables during basic and detailed design and execution were implemented in the same tried and tested way by engineering, suppliers, and biopharmaceutical companies. Making decisions was complicated by alignment with long lead items. With the introduction of single-use (SU) technologies, the transition in execution and optimization of project management only just has been…