Chimeric antigen receptor (CAR) T cells first entered US clinics in 2017 (1), and this therapeutic modality holds tremendous potential as one of the most effective forms of personalized cancer care ever to reach patients. The revolutionary impact of CAR T-cell therapy comes from its ability to rewire our own immune defenses to kill cancer cells: It essentially modifies a patient’s naturally existing immune cells to boost their recognition and attack of cancer cells so that the person’s own immune system…
Manufacturing
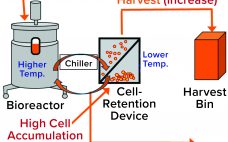
Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits:…
Practical Considerations for Statistical Analyses in Continued Process Verification
Several statistical techniques can be used to assist in monitoring biopharmaceutical product quality attributes as part of continued process verification (CPV) activities. These include run charts, control charts, and capability analyses. Below, I provide an overview and recommendations on statistical strategies when developing a CPV program, considering the expected behavior of manufacturing results in the biopharmaceutical industry. Presence of Autocorrelated Data In a previous study, I highlighted the tendency for data to be positively autocorrelated (values are closely related to…
Analytical Support for Biologics: A Conversation with Almac Sciences
Almac Sciences (a member of the Almac Group) recently expanded its suite of analytical solutions to include biologics testing. This follows a 2019 expansion of the company’s facility in Athlone, Ireland, where it provides a comprehensive range of flexible pharmaceutical testing services to support drug development programs adhering to industry regulations and good manufacturing practice (GMP) standards. “Biologics have gained huge traction in the past decade and are poised for stronger growth in the coming years with potential to significantly…
Ultrasonic Flow and Bubble Sensors: Optimize Process Quality in Single-Use Bioprocessing Applications
Process monitoring in laboratory and production environments enables continuous control and optimization of critical process parameters. Hence, the early detection of errors is an effective means of increasing process efficiency and reproducibility, improving quality and safety parameters, and reducing long-term costs. Highly precise and flexible noncontact clamp-on flow and bubble sensors are useful instruments to fulfill these goals. They can be applied effectively to buffer and media preparation, chromatography and filtration systems, bioreactors and fermentors, feed and transfer lines, and…
Evolving Business Models to Meet the Future of Healthcare
Yourway provides integrated supply-chain solutions for the global pharmaceutical and biotechnology industries. We exceed traditional offerings to provide customized support that includes warehousing and packaging, project management, planning and optimization guidance, comparator sourcing, ancillary-supply sourcing, forecasting, and returns/reconciliation management. Our single–specialized-provider approach offers clients a high level of convenience and efficiency that translates into quality, speed, and operational improvements. A Growing Global Footprint As we introduce new added-value solutions, expand our global footprint, and continue investing in infrastructure, technology, training,…
Case Study for a Facility-Fit Driven Process Development
Time to clinic and time to market are the key drivers for client success in the biopharmaceutical industry. Facility fit is becoming key to understanding process constraints and which aspects of the process have the largest impact on enabling facility fit. Process development with facility-fit constraints in mind will ensure a smooth technology transfer and shorten the timeline of current good manufacturing practice (CGMP) product delivery to clients. Fill out the form below to read this Special Report and learn…
Cryopreserved Leukapheresis Materials Help Alleviate Donor Sourcing Issues
Reliable access to high-quality starting material is a primary challenge in cell therapy manufacturing. Freshly isolated leukopak starting materials depend on donor availability and are vulnerable to cell losses from scheduling changes and other unforeseen circumstances. Flexibility often is required for cell therapy manufacturing. Therefore, relying solely on freshly isolated starting material is impractical, particularly when cell therapy logistics involve global shipping and distribution. Donor sourcing is among the most critical factors shaping cell and gene therapy (CGT) supply chains.…
Innovations in the Manufacturing of Cell and Gene Therapies
Advancements in next-generation cell and gene therapies are fulfilling the promise of personalized medicine and attempting to cure and heal patients. Multiple approved products have been launched in global markets and the number of clinical trials continues to grow. Developing innovative advanced therapies is one of the biopharmaceutical industry’s greatest opportunities to dramatically improve patients’ lives. WuXi Advanced Therapies is a contract testing, development, and manufacturing organization (CTDMO) that has launched several innovative world-class platforms. It offers integrated platforms to…
eBook: Drug Delivery —
Partnerships Are Key As Medical and Digital Worlds Converge
Drug delivery is advancing into a digital future. Information technology is changing aspects of every operation in the biopharmaceutical industry. Meanwhile, machine learning and cloud computing are not only finding their way into drug development, manufacturing, and distribution, but also into drug-product delivery devices themselves. As a result, many biopharmaceutical companies are seeking medical device expertise through strategic alliances and contract services. New delivery devices can help big companies extend patent protection on established marketed products, for example, and provide…