Rapidly growing interest in gene therapy has led to the need for more cost-effective and scalable viral-vector manufacturing platforms. Adenoassociated virus (AAV) has become a vector of choice because of its safety profile (nonpathogenic infection). In addition, AAV cannot replicate on its own and is not integrated directly into the host genome. AAV vector manufacturing using human embryonic kidney (HEK) cells in either adherent or suspension mode includes several typical processing steps: cell expansion, plasmid transfection, viral-vector production, cell lysis,…
Manufacturing
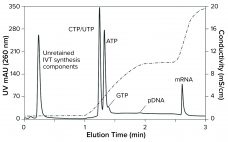
Eliminating the Analytical Bottleneck in Production and Purification of mRNA
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…
Space Travel Rockets Mitochondria’s Central Role into the Spotlight
Mitochondria originated millions of years ago, likely when a eukaryotic cell incorporated a bacterium. Thus, mitochondrial DNA strands are 200,000Ă— smaller than those from a cell’s nucleus. Depending on cell type, thousands of mitochondria can reside within the walls of one human cell. The scientific community long has considered these organelles to be cellular “energy factories.” But their centrality to human health and disease only now has come to light. In a breakthrough study published in Cell (1), biologists collaborated…
Eradicating the Need for Cold-Chain Distribution in the Biopharmaceutical Industry
Cold chain distribution is complicated and critical for formulations that must be kept in very cold temperatures in the pharmaceutical industry, since their stability decreases quickly at room temperatures. The World Health Organization (WHO) has reported over 50% of vaccines are wasted and must be disposed of globally every year due in part to disruption of the cold chain distribution and lack the resources to support the ultracold temperature requirements. A possible solution to the existing problem is Hyalo Technologies’…
The Five Heresies of Cell Culture: Debunking Conventional Wisdom
Cell culture and bioprocessing conventional wisdom remains a hurdle for the wider adoption of more precise tools. It has been more than 60 years since any real progress has been made towards creating a more accurate and reliable way of performing cell culture monitoring to better understand the effects of things like pH and oxygen at the pericellular level. At SBI, we’re developing optical sensing technologies that unlock the “black box” of cell culture to bring actionable insights to scientists…
Measuring Cell Density in HyPerforma S.U.B.s with ABER Futura neotf
Single-Use Sensors
Monitoring critical process parameters (CPPs) and key performance indicators in bioreactor control systems is crucial to ensure proper cell growth and protein production. Today, most of the major biopharmaceutical companies employ capacitance measurement, in R&D and through process development to manufacturing. Owing to the increased use of single-use bioreactors and building on Aber’s experience with single-use capacitance sensors, the latest Futura neotf single-use capacitance sensors have been specifically developed for integration into Thermo Fisher Scientific bioprocess containers (BPCs) for use…
Ask the Expert: Selecting the Right Buffer Management Strategy
Although buffers are among the simplest materials used in bioprocessing, they are critical to biopharmaceutical manufacturing success. Buffer preparation, storage, and handling can require significant investments in time, labor, equipment, and facility space. Jenny Dunker, MSc, and Alexander Troken, PhD (global product managers for customized bioprocess solutions and for process liquids and buffers, respectively, at Cytiva), delivered an “Ask the Expert” presentation on 30 March 2021 to explore strategies for intensifying buffer management. Available Options Biopharmaceutical manufacturers often prepare buffers…
Single-Use Systems for Storing and Shipping Frozen Drug Materials
Using presterilized, single-use freeze–thaw systems instead of traditional freeze–thaw platforms that include stainless-steel tanks and bottles can help biomanufacturers manage the quality of their drug substances. Single-use assemblies reduce the risk of cross-contamination, simplify dispensing, and decrease the number of manual interventions during freezing, thawing, handling, and shipping. However, implementing a freeze–thaw process requires careful testing of the physical and thermal properties of single-use systems and related aseptic connectors as well as assessment of drug-substance quality and stability. Such evaluation…
Cell-Free Expression: A Technology with Truly Disruptive Potential
Bioprocess engineer Beatrice Melinek is a postdoctoral research fellow at University College London’s Future Targeted Healthcare Manufacturing (FTHM) Hub, where she focuses on the use of cell-free protein synthesis (CFPS) as a platform for distributed production of stratified biotherapeutics. Previously Melinek specialized in purification of viral vectors and vaccines, with an engineering doctorate (EngD) in biochemical engineering and postdoctoral experience in UCL’s hematology department developing a new chromatography-based analytical method for measuring empty and full adenoassociated virus (AAV) capsids. She…
eBook: ADCs — Evolving Links in the Biopharmaceutical Pipeline
Antibody–drug conjugate (ADC) developers both old and new are talking about the next generation of drug candidates coming through their pipelines. In April 2021, Zynlonta (loncastuximab tesirine, from ADC Therapeutics) became the eleventh such product to receive approval from the US Food and Drug Administration (FDA). But with dozens of ADC candidates currently in clinical trials, those 11 products represent the tip of the ADC iceberg. In this eBook, Dan Stanton (founding editor of BioProcess Insider) explores ADC production history,…