Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development. Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Although interest in vaccine development and manufacture continues to increase because of the rapid growth of the global vaccine market, this area of the bioprocess industry remains challenging and complex. Here we review the current constraints and complexities in the vaccine industry, specifically related to product development and manufacture. We…
Continuous Bioprocessing
Continuous Biomanufacturing: A New Approach to Process Scale
The BioPhorum first-edition Technology Roadmap outlined a 10-year vision for therapeutic protein production in the biopharmaceutical industry (1). The roadmap describes multiple manufacturing scenarios ranging from large-scale (~20,000-L production) to small and agile, portable production facilities. It includes detailed analyses of the needs for the future in each of the following areas: Process technologies (2) Inline monitoring and real-time release (3) Automated facilities (4) Modular and mobile (5) Knowledge management (6) Supply partnership management (7). Since the 2017 publication of…
From Interest in Intensification to a Factory of the Future
Much has been published on improvements and advances in many individual technologies for biomanufacturing. If you take a comprehensive look at the field, however, you find overlap, muddling, and even contradiction about which particular processes or aspects of technological development should be designated properly as process intensification. Although the industry is addressing such distinct goals as improved manufacturing yield, product quality, and cost-effectiveness, the names of initiatives commonly applied to accomplish those goals overlap at best. Such ambiguity and lack…
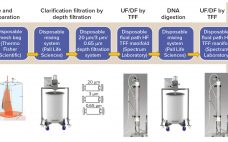
An Integrated Bioprocess for Antibodies: From Harvest to Purified Bulk in Six Hours
Antibody production platform processes have been widely adopted in biomanufacturing, but many unit operations are not suitable for integration and automation. Here we describe the work of integrating unit operations by transforming a column operation to a more robust cassette format. We have selected a biomolecule-friendly buffer (phosphate) to eliminate, or delay, the performance of a circulating tangential flow ultrafiltration/diafiltration (UF/DF) operation, so the harvest-to-purified-bulk process can be integrated, resulting in a single, direct-flow operation, that reduces the batch process…
Making Downstream Processing Continuous and Robust: A Virtual Roundtable
Current biomanufacturing is driven to pursue continuous processing for cost reduction and increased productivity, especially for monoclonal antibody (MAb) production and manufacturing. Although many technologies are now available and have been implemented in biodevelopment, implementation for large-scale production is still in its infancy. In a lively roundtable discussion at the BPI West conference in Santa Clara, CA (11 March 2019), participants touched on a number of important issues still to be resolved and technologies that are still in need of…
On Continuous Chromatography: A Conversation with Sanofi’s George Weeden
George S. Weeden, Jr., is a scientist in global manufacturing science and technology (MSAT) process science at Sanofi. We recently chatted about the topic of continuous chromatography. What are the general reasons for companies to consider continuous chromatography? And what are the caveats? The main driver for considering continuous chromatography is reducing the cost of goods (CoG). Continuous chromatography improves productivity (mass of product per volume of stationary phase over time) and thus increases throughput or decreases volumes of stationary…
Continuous Chromatography: Experts Weigh in on the Possibilities and the Reality
Discussions of continuous processing in the biopharmaceutical industry are an important part of current efforts toward intensifying bioproduction and bioprocessing. Biomanufacturers are looking at all components of their development and manufacturing processes for ways to reduce the size of their facilities, lower costs, and increase speed and flexibility of operations. Increasing options for and availability of single-use technologies have been major enablers of myriad attempts to improve efficiencies. Although the general consensus may still be that single-use components are more…
In-Line Turbidity Sensors for Monitoring Process Streams in Continuous Countercurrent Tangential Chromatography (CCTC)
A strong connection between turbidity and total suspended solids (TSS) has been linked in the past to measuring well defined particles in processes. Optical density probes have seen wide adoption in the biotechnology industry for monitoring cell growth within a bioreactor, whereas in-line turbidity sensors have been used to monitor filter performance. Turbidity measurements offer a rapid quantification of suspended solids but have not been used in the biotechnology industry for chromatographic resins. In this study, turbidity measured with equipment developed by PendoTECH was used with novel continuous chromatography technology developed by Chromatan…
Accelerating Intensified Bioprocesses with High-Throughput Small-Scale Tools
While many biopharmaceutical companies are exploring paths toward continuous processing, many tools already exist for implementing process intensification. As the authors of this special report illustrate, hybrid continuous processes that benefit from single-use technologies along with continuing improvements in perfusion cell culture already now are enabling improvements in cost reduction and accelerating time to market. And novel high-throughput and automated small-scale systems are helping development scientists gather more information in less time than before, reduce their development footprints, and make…
Data Science, Modeling, and Advanced PAT Tools Enable Continuous Culture
Bioprocesses traditionally use (fed-)batch cell culture processes for production of recombinant proteins and therapeutics. In batch bioprocessing, material flow is discrete, with a hold step between two unit operations, and product is harvested only once for each unit operation. Batch processes have been studied extensively and optimized through numerous advancements in experimental design (1, 2), monitoring (3–5), measurement techniques (6–9), and control strategies (10–12). However, such processes require large facility footprints for equipment (13) as well as sterilization, load, and…