Steadily increasing demand for biopharmaceutical drugs has led the industry to examine its manufacturing scales while pressuring research and development groups to produce high-yielding clones and processes. Improved media, feed supplements, bioreactor designs, and control of process parameters have helped biomanufacturers achieve multifold increases in volumetric productivity from production bioreactors. However, cell culture processes are significantly affected by their bioreactor’s ability to support cells at higher densities and sustain cultures at lower viabilities. With the implementation of a number of…
Filtration
Downstream Disposables: The Latest Single-Use Solutions for Downstream Processing
Downstream processing has been considered a “bottleneck” in the manufacture of protein biotherapeutics ever since cell culture engineers began dramatically improving production efficiencies around the turn of the century. And as single-use technologies have grown in importance and acceptance, offering more solutions every year, their biggest challenges too have been in the separation, purification, and processing that follows product expression in cell culture. Many of the technologies familiar to process engineers — e.g., centrifugation and chromatography — present technical and…
Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures
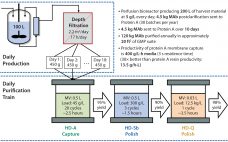
For current process development phases, many biomanufacturers’ attention is directed increasingly to the first unit operation in downstream processing, which is the removal of cells and cell debris from culture broth and clarification of supernatant containing a biopharmaceutical product. Given the high cell densities achievable with both mammalian and microbial cell culture processes, primary recovery can be a significant challenge. The current trend in cell culture is to increase product titers with enriched culture media, improved cell productivity, and increased…
Membrane Adsorbers, Columns: Single-Use Alternatives to Resin Chromatography
Filtration membranes are used extensively throughout the biopharmaceutical industry for a range of applications, from coarse filtration to nanofiltration. Advantages of filter technologies include easy scaling, disposability, and (for many membrane filters) rapid and robust performance in a single-pass. The same advantages have been realized with membrane adsorbers. Chromatography resins are inherently disadvantaged by diffusion limits of the pores in chromatography media. Therefore, resin columns must be significantly oversized to match the performance of high productivity bioreactors. By comparison, membrane…
Membrane-Based Clarification of Polysaccharide Vaccines
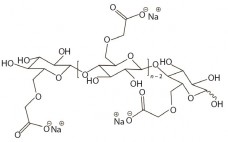
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a Haemophilus influenzae type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (1). Polysaccharides are relatively complex carbohydrates made up of many…
Best Practices for Critical Sterile Filter Operation: A Case Study
A number of regulatory guidelines recommend preuse integrity testing of critical sterilizing liquid filters for aseptic processing (1–3). Before sterilization, a preuse test will confirm that a filter is installed properly and was not damaged during shipment or handling. Performing a preuse test after sterilization detects damage that may have occurred during the sterilization cycle. Testing after sterilization limits risk, so it is a practice applied based on risk assessment. Because it is perceived to reduce business loss risk, preuse…
Virus-Filtration Process Development Optimization: The Key to a More Efficient and Cost-Effective Step
Size-exclusion–based parvovirus filtration is an important step toward drug product safety in biopharmaceutical production. However, once a virus filter is in place, and the required virus safety is ensured, less attention typically is paid to its optimization within the process. That might seem odd given that virus filtration can be one of the more expensive downstream processing steps ($/g protein processed). Most likely, the lack of attention can be attributed to aggressive timelines, limited process development resources, and the virus…
Factors Affecting Sterile Filtration of Sodium-Carboxymethylcellulose–Based Solutions
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (1–3), serves as a suspension aid (4), and stabilizes emulsions (5). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (6, 7). CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree…
Characterization of Postcapture Impurity Removal Across an Adsorptive Depth Filter
In the manufacture of monoclonal antibodies (MAbs), the first purification step following harvest clarification is normally protein A affinity chromatography because of its high selectivity for IgG and high process yield (1, 2). At this stage, a MAb is eluted from a protein A ligand at low pH and then held or adjusted to a low pH (pH ≤ 3.8) for a given amount of time before pH adjustment, usually ≥30 minutes, in a virus inactivation (VI) step targeted at…
Evaluating Adsorptive Filtration As a Unit Operation for Virus Removal
To date, the majority of recombinant monoclonal antibodies (MAbs) have been produced by mammalian cells. During such production processes, the potential risk of entrained viruses must be critically considered (1). Contamination can arise from animal cell lines or from adventitious viruses introduced during manufacturing. To ensure the viral safety of biotechnology products, companies can take four complementary approaches (2, 3): Using animal-component–free raw materials wherever possible Virus testing of master cell banks Virus testing of unprocessed harvest Performing downscale virus…