In the past year, CPhI Worldwide (a division of UBM EMEA) has released reports on both the Indian and Chinese pharmaceutical markets, findings of which were presented at CPhI India in Mumbai in early December 2014 and shortly after CPhI China in August 2015. India Looking Outward to Innovate The Indian report, CPhI India Pharmaceuticals 2015: Industry Explorations, was developed by CPhI in partnership with Global Business Reports (GBR) to provide a comprehensive analysis of the country’s pharmaceutical market. Overall, Indian companies…
Economics
Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 2: Clinical Efficacy, Reimbursement, and Needle-to-Needle Logistics
Cell therapy is an emerging pillar in healthcare with the potential to provide curative solutions to a wide range of indications. The biological complexities through which cell technologies exert their clinical impact (especially those used in immunotherapies for cancer) provide opportunities for novel modes of immune regulation, cell targeting, and payload delivery. Cells also can serve as vehicles for genetic content, which the gene therapy industry is now investigating. Since early 2004, Invetech has worked with organizations dedicated to cell…
Bioreactor Design for Adherent Cell Culture: The Bolt-On Bioreactor Project, Part 4 — Process Economics
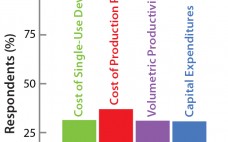
The Bolt-on Bioreactor (BoB) project is an independent initiative developing and commercializing a bioreactor for efficient, automated culture of adherent cells for biopharmaceutical applications (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges: volumetric productivity (2), process automation (3), containment and sterility (4), and process economics. This month concludes a four-part series addressing each of those challenges while describing design features…
Special Report: A World of Difference — Biosimilars and Biobetters Offer Unique Benefits — and Risks
by John Otrompke, with Cheryl Scott and S. Anne Montgomery When the United States Food and Drug Administration (FDA) approved the country’s first ever biosimilar on 6 March 2015, it had been a long time coming. After all, the European Union had approved the first biosimilar in 2006, and a number of others have followed in Europe since then. Still, the approval of biosimilar filgrastim, a recombinant colony-stimulating factor used to offset the complications of chemotherapy, was a welcome step…
Paying for Pricey Medications: Debt Financing Options Could Provide a Solution
In an era of US$1,000/dose medications, a new approach may be needed to finance an emerging breed of expensive but highly effective pharmaceuticals and vaccines, according to a new Rand Corporation analysis. In other industries, it is common for suppliers to encourage customer investment — particularly for costly capital purchases such as new automobiles and machinery — through approaches such as equipment leases or supplier-financed credit. The healthcare industry could learn from such approaches. For example, instead of paying up…
To Serve and Promote: A Conversation with BIO’s President and CEO
BIO is the world’s largest trade association representing biotechnology companies, academic institutions, state biotechnology centers and related organizations across the United States and in more than 30 other nations. BIO members are involved in the research and development of innovative healthcare, agricultural, industrial, and environmental biotechnology products. BIO plays a leading role in shaping public policy related to the biotechnology industry — at the state, national, and international levels. Jim Greenwood has been BIO’s president and CEO for 10 years,…
Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 1: Achieving Manufacturability and Managing Cost of Goods
Much mystique and mystery surround the emerging industries of cell therapy and regenerative medicine. As companies progress toward commercial manufacture with potential game changers (e.g., cures for cancer and diabetes) the industry could be on the verge of significant breakthroughs. However, with no real successes to date, the question is raised: What core attributes are required to achieve commercial success? The tale of Dendreon’s struggles highlights how difficult it can be to commercialize even an approved cell therapy product. Since…
When Is a Virtual Business Model Suitable for Biopharmaceutical Companies?
Virtual companies are based on the model that all activities are outsourced. Such companies have no (or few) employees, occupy no laboratory space, and use contract service organizations for all activities. Over the past decade, several virtual biopharmaceutical companies have formed (1–4). They are primarily start-up ventures that use contract research organizations (CROs) for R&D and contract manufacturing organizations (CMOs) for product manufacturing. By contrast, a fully integrated biopharmaceutical company is based on the model that all activities are internal to…
A Quick Guide for Sourcing Biopharmaceutical Raw Materials
Before the ratification of regulatory guidelines from The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q8–Q11 (1–4) — whose scope includes raw materials for biopharmaceutical production — many drug manufacturers chose the most cost-effective and readily available raw materials sourcing options without specifically considering the provenance of those materials. Depending on the chosen supply chain, such materials could be of widely varying quality and not necessarily suitable for a destined application. Raw-material…
A Case for Biopharmaceutical Operations in Low-Cost Countries to Maximize Return on Investment
A company’s success is associated with its growth. An expanding company hires more employees (full-time equivalents, FTEs) to support its commercial products, to develop new products, and to provide administrative services. As it grows, an organization can become more complex and its operations less efficient. Analysis of 2013 financial data showed that revenue of US biopharmaceutical companies increases with a growing number of FTEs at a slower rate than does cost (1). That study assumed that all US biopharmaceutical companies…