Presented by: Noel Smith, head of immunology, Lonza Pharma & Biotech Biopharmaceutical developers are keenly aware of high attrition during preclinical drug development stages. Immunogenicity and immunotoxicity concerns account for many program failures. Thus, developers strive to identify safety concerns as early as possible. By detecting problems early, companies can reengineer their molecules, investigate process-related solutions, or select alternative lead candidates. Smith reviewed methods that Lonza has developed to enhance early immunogenicity and immunotoxicity studies. Smith noted that several factors…
Manufacturing
Key Challenges and Potential Solutions for Eliminating Bottlenecks and Optimizing Downstream Operations
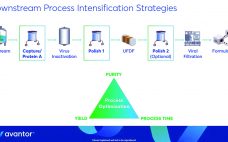
Presented by: Willie Hesselink, technical application project manager, biopharmaceutical production, Europe, Avantor Investments in cell-culture technologies have increased monoclonal antibody (MAb) yields significantly over the past two decades. Downstream operations continue to lag in productivity, however. Hesselink explored how companies can align downstream productivity with upstream yields to mitigate production bottlenecks. He focused on intensifying chromatography steps, optimizing buffer preparation, and mitigating variations across raw materials. Chromatography resin selection is vital to downstream intensification, Hesselink explained. Capture and polishing steps…
A Behind-The-Scenes Look at Lykan Bioscience’s State-of-the-Art, Purpose-Built Cell Therapy Facility
Presented by: Patrick Lucy, president and chief executive officer, Lykan Bioscience Lykan Bioscience is a cell-therapy–focused contract development manufacturing organization (CDMO) based in Hopkinton, MA. Lucy’s presentation showcased his company’s new purpose-built manufacturing facility and described how its design can help to expedite manufacturing of cell therapy products. Emphasizing the highly individualized nature of autologous therapy production, Lucy also explained how the Lykan team integrates its logistical and manufacturing operations to maximize patient access to lifesaving treatments. Lykan’s 64,000-ft2 facility…
BioProcess Insider Interview: Sam Machour, Samsung Biologics
BioProcess Insider brings the biotechnology news as it breaks. For the on-demand BPI Theater at the Biotechnology Innovation Organization’s 2021 convention, founding editor Dan Stanton interviewed leading biopharmaceutical executives in early June 2021. Contract development and manufacturing organizations (CDMOs) have thrived during the COVID-19 pandemic despite constraints on supply chains. Sam Machour, senior vice president and chief quality officer at Samsung Biologics explained that his company’s pandemic-period success has stemmed primarily from its ability to accelerate production timelines. Before 2020,…
CGMP Manufacturing Facility Design and Evolution
Presented by: Mike Alston, Jr., director, project engineering; and William Leonardi, PhD, senior project manager, Avid Bioservices These two speakers shared lessons learned in the design and buildout of current good manufacturing practice (CGMP) manufacturing facilities at Avid. Leonardi began with a history of the company’s 28+ years of biologics development and manufacturing and its track record as a successful contract and development manufacturing organization (CDMO). Avid’s main campus comprises a cluster of buildings in Tustin, CA. GMP, process-development, and…
Preparing for Preapproval Inspection (PAI): How toTransition Operations from Early Stage to Phase 3 and Commercial Production
This podcast features: Patricio Massera, general manager of the Copenhagen facility for CMC Biologics This podcast was recorded at the 2014 BioProcess Theater which took place at the 2014 BIO International Convention, June 23-26, 2014. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and scientific trends impacting…
Product Development Partnership: AERAS as a Model
This podcast features: Peter Alexander, senior director of technical operations at AERAS This podcast was recorded at the 2014 BioProcess Theater which took place at the 2014 BIO International Convention, June 23-26, 2014. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and scientific trends impacting the development…
Developing a Robust and Scalable CGMP Protein Manufacture Process
This podcast features: Daniel Smith, PhD, chief scientific officer of Cobra Biologics, and Julian Hanak, commercial director of Cobra Biologics This podcast was recorded at the 2014 BioProcess Theater which took place at the 2014 BIO International Convention, June 23-26, 2014. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the…
The Future of Aseptic Filling: Automated Filling Robots Coming to a Clean Room Near You
This podcast features: Gene Yoshioka, director of manufacturing at Avid Bioservices This podcast was recorded at the 2014 BioProcess Theater which took place at the 2014 BIO International Convention, June 23-26, 2014. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and scientific trends impacting the development and…
CMO/CDMOs: How Technologies Are Changing Outsourcing Strategies, Services Offered, and Risks Taken
This podcast features: Moderator: Susan Dexter, principal consultant at Latham Biopharm Group Panelists Economic Trends: Rob Silber, director of supply chain/business contracts at Boehringer Ingelheim Technology Advancements: Andrew Sandford, vice president of business development at Catalent Pharma Solutions Facility Designs: Bill Reed, PhD, senior vice president of operations at Kalon Biotherapeutics Service Offerings: Mark Bamforth, president and chief executive officer at Gallus BioPharmaceuticals This podcast was recorded at the 2014 BioProcess Theater which took place at the 2014 BIO International…