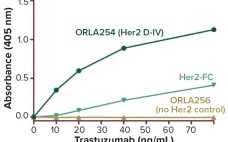
Monoclonal antibodies are a rapidly growing class of therapeutics and are used in multiple clinical indications. This highly competitive landscape necessitates the need for rapidly developing next generation antibodies against new challenging targets with improved mechanism of action and pharmacokinetics. Current antibody screening tools such as ELISA, only report on binding to one antigen target at a time; hence these assays are time consuming and require large amounts of target protein. This case study highlights how the Intellicyt® iQue3 system…
Analytical
Taking Your Molecule Through Process Validation
The dynamics of the biopharmaceutical industry to get innovative products to the client has evolved over the years. Studies have shown that by 2021, biologics and biosimilar products are projected to have higher growth than other pharmaceutical products. Following the industry trend, Avid Bioservices as a Contract Development Manufacturing Organization (CDMO) has helped numerous clients complete their process validations campaigns. Between 2016 to 2019, Avid has successfully completed six. This custom report will share some key factors to consider for…
Biopharmaceutical Characterization, Part 2: Applications and Strategies for Diverse Products — A Conference Report
Last fall, KNect365 brought together more than 250 analytical specialists to discuss biological assays and characterization of well-characterized biologics in Rockville, MD. Speakers from the US Food and Drug Administration joined experts from leading biopharmaceutical companies, service providers, and consultancies for case studies, regulatory interactions, sharing perspectives, and learning about emerging technologies. Part 1 of this report in January 2019 focused on the bioassay section of the meeting. Here in Part 2 sponsored by Sartorius, BPI’s senior technical editor reports…
Analytical Challenges: Characterization of Oligonucleotide Therapeutics
Recent approvals of oligonucleotide therapeutics are a clear signal for optimism for this product class. This is supported by the strength of the current pipeline which has over 180 active oligonucleotide clinical programs in various phases of development. Improvements in analytical technology and know-how have played a key role in enabling suitable characterization and quality control strategies to overcome the difficulties associated with testing these complex molecules. Despite the lack of dedicated regulatory guidelines related to characterization or quality control,…
Analytical Tools to Improve Decision-Making During Product Development
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis…
Modeling Virus Clearance: Use of a Noninfectious Surrogate of Mouse Minute Virus As a Tool for Evaluating an Anion-Exchange Chromatography Method
Viral safety is a critical focus during biopharmaceutical manufacturing (1–5). Although well-characterized mammalian cells such as the Chinese hamster ovary (CHO) line have been used for decades, both endogenous expression of retroviral-like particles and exogenous contamination events from viruses warrant continued vigilance (6, 7). International regulatory agencies require biomanufacturers to validate the “viral clearance” efficacy of their downstream manufacturing process steps before resulting products can be awarded clinical trial or commercial approval (8–10). Currently, viral clearance testing is based on…
Integrity Redefined: Consistent Robustness and Integrity Testing Lead to Enhanced Process Integrity and Patient Safety
With the increasing adoption of single-use systems (SUS) in critical stages of biopharmaceutical manufacturing, any lack of system integrity can significantly affect drug product quality and patient safety, as well as incur additional costs due to product loss and disrupted production cycle. This article from Sartorius Stedim Biotech, describes how determining the correlation between liquid leakage and microbial ingress can be used to define MALLs (Maximum Allowable Leakage Limits) of SUS for different process steps. The article also details the…
Improving CHO Cells for Biomanufacturing
Chinese hamster ovary (CHO) cells have been used in biomanufacturing for decades because of their robust capacity to express a range of proteins, such as therapeutic enzymes and monoclonal antibodies (MAbs) at titers measured in multiple grams per liter of culture. Within the available suite of CHO cell lines, the glutamine synthetase knockout (GS-KO) selection system provides industry-leading speed to the identification of high-producing clones for use in biomanufacturing. The GS-KO selection system allows for identification of multiple-gram/L clones in…
Visible Particulate Matter in Single-Use Bags: From Measurement to Prevention
Parenteral pharmaceuticals must be “essentially free” from visible particulate matter (1). In the production of biopharmaceuticals with single-use systems (SUS), biocompatibility requires controlling interactions between drug substances/products and SUS surfaces to ensure drug product quality and patient safety with regard to extractables/leachables and particulate matter. Any particulate matter stuck to fluid-contacting surfaces of process components could wash off and contaminate process fluids. Depending on system configuration, a final drug product could be at risk for particulate matter from SUS. Risk…
Monoclonal Antibodies: Beyond the Platform in Manufacturing
The vast majority of monoclonal antibody (MAb) production processes are based on fed-batch Chinese hamster ovary (CHO) cell culture and protein A affinity column chromatography capture. Increasing cost-consciousness — among innovator companies as well as biosimilar makers — has many companies looking “beyond the platform” for less expensive alternatives that may provide better results. Here the BPI editors review some state-of-the-art alternatives in upstream and downstream MAb drug substance bioprocessing as well as drug-product manufacturing. The current “gold standard” platform…