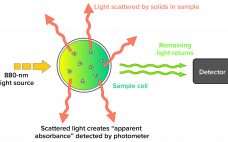
Turbidity describes the relative clarity of a liquid as the result of suspended solids. Instruments that measure turbidity typically use a beam of light to detect particles by measuring the difference between the amount of light emitted from the light source and the amount that is received by a detector. Such measurements are affected by the size, shape, and number of particles in a sample of liquid because those solids scatter the incoming light, which provides an apparent absorbance that…
June 2021
Overcoming Obstacles in AAV Viral Vector Manufacturing
Rapidly growing interest in gene therapy has led to the need for more cost-effective and scalable viral-vector manufacturing platforms. Adenoassociated virus (AAV) has become a vector of choice because of its safety profile (nonpathogenic infection). In addition, AAV cannot replicate on its own and is not integrated directly into the host genome. AAV vector manufacturing using human embryonic kidney (HEK) cells in either adherent or suspension mode includes several typical processing steps: cell expansion, plasmid transfection, viral-vector production, cell lysis,…
Eliminating the Analytical Bottleneck in Production and Purification of mRNA
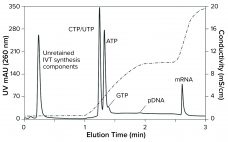
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…
Space Travel Rockets Mitochondria’s Central Role into the Spotlight
Mitochondria originated millions of years ago, likely when a eukaryotic cell incorporated a bacterium. Thus, mitochondrial DNA strands are 200,000Ă— smaller than those from a cell’s nucleus. Depending on cell type, thousands of mitochondria can reside within the walls of one human cell. The scientific community long has considered these organelles to be cellular “energy factories.” But their centrality to human health and disease only now has come to light. In a breakthrough study published in Cell (1), biologists collaborated…
Eradicating the Need for Cold-Chain Distribution in the Biopharmaceutical Industry
Cold chain distribution is complicated and critical for formulations that must be kept in very cold temperatures in the pharmaceutical industry, since their stability decreases quickly at room temperatures. The World Health Organization (WHO) has reported over 50% of vaccines are wasted and must be disposed of globally every year due in part to disruption of the cold chain distribution and lack the resources to support the ultracold temperature requirements. A possible solution to the existing problem is Hyalo Technologies’…
Ask the Expert: Selecting the Right Buffer Management Strategy
Although buffers are among the simplest materials used in bioprocessing, they are critical to biopharmaceutical manufacturing success. Buffer preparation, storage, and handling can require significant investments in time, labor, equipment, and facility space. Jenny Dunker, MSc, and Alexander Troken, PhD (global product managers for customized bioprocess solutions and for process liquids and buffers, respectively, at Cytiva), delivered an “Ask the Expert” presentation on 30 March 2021 to explore strategies for intensifying buffer management. Available Options Biopharmaceutical manufacturers often prepare buffers…
Ask the Expert: Considerations for Mass Spectrometric HCP Analysis During Process Development
Host-cell proteins (HCPs) must be assessed early in biopharmaceutical product development to establish the efficiency of a purification process. Liquid chromatography coupled with mass spectrometry (LC-MS) is a strong orthogonal method for HCP identification and quantitation. During an 18 March 2021 “Ask the Expert” presentation, Christina Morris (senior scientist at BioPharmaSpec) explained how MS analyses can enhance process development (PD), then presented considerations for generating spectral libraries, selecting samples for quantitation, and reviewing data. Morris’s Presentation Spectral Libraries: A typical…