BPI’s June issue always precedes a break in our regular issue schedules in July and August. But summer does not afford us any leisure. This July, we again shall bring you an eBook of summaries from the BPI Theater at the Biotechnology Innovation Organization’s annual convention (this year from digital presentations, which will be available in full on the BPI site) along with our annual Industry Innovators issue. Summer eBook topics will be formulation, exosomes, biosimilars, and expression systems. BPI’s…
June 2020
The Green Imperative: Part One — Life-Cycle Assessment and Sustainability for Single-Use Technologies in the Biopharmaceutical Industry
Much has changed since large-scale single-use biomanufacturing equipment was introduced some 15 years ago. Since then, these materials have become accepted and established in production and downstream bioprocessing. Concerns about the environmental impact of single-use (SU) biomanufacturing equipment have become more prevalent as our environmental awareness has increased and related concerns have become more urgent (1). For example, many recommendations and even laws have emerged regarding plastic convenience packaging and products (2, 3). People have become more sophisticated in appreciating…
Applications of Disposable Technologies for Upstream Bioprocessing
Over the past 10 years, a number of developments in disposable (limited use) and single-use technologies (SUTs) have been made for different bioprocess operations. Until recent years, much of the industry’s process equipment was sterilized using thermal methods such as autoclaving. Most equipment was reusable and required cleaning and sterilization before use. Such processes required validation and expensive and time-consuming resources. Production facilities relied on hard-piped, inflexible equipment such as large stainless-steel bioreactors and holding tanks. However, advanced SUTs now…
Life-Science Lawsuits: Learning from the Ordeal
Life-science companies often are cast into the role of the “canary in the coal mine” — the first parties to be targeted and hit by lawsuits. Such companies depend on discovery, trial and error, and ultimately efficacy. None of that is a sure bet. At the same time, life-science companies are raising funds constantly to finance their work. Investors and lenders seeing less-than-projected or even “expected” results might sue directors and officers for mismanagement, misrepresentation, or misleading financial statements. This…
Trends in COVID-19 Diagnostic Test Development
The ongoing coronavirus disease 2019 (COVID-19) outbreak caused by a novel coronavirus (SARS-CoV-2) is posing a great threat to global public health and economies. Accurate and rapid detection of the SARS-CoV-2 virus and diagnosis of infection status will play a critical role in understanding the disease, selecting appropriate treatments, controlling the spread, and developing informed back-to-work policies. Currently, a review of ongoing efforts in the development of COVID-19 diagnostic tests is lacking. For this review, we collected data on more…
Viral-Vectored Gene Therapies: Harnessing Their Potential Through Scalable, Reproducible Manufacturing Processes
We might not associate the jazz queen Ella Fitzgerald with 21st-century gene-based therapies, but the First Lady of Song was on to something back in 1939 when she sang “’T’Ain’t What You Do (It’s the Way That You Do It).” Although demonstrating the safety and efficacy of gene-based therapies in rigorous clinical trials is essential for gaining product approval from regulators, doing the bare minimum is insufficient. The way that such products are produced also matters. Manufacturing processes and protocols…
Anticipating Cell-Line Challenges to Drive CMC Readiness
Development of a safe and high-quality Chinese hamster ovary (CHO) cell line is of paramount importance for the chemistry, manufacturing, and controls (CMC) portion of studies that support investigational new drug (IND) applications (1, 2). Desirable attributes of a CHO cell line include its ability to produce high titers of biotherapeutic proteins facilitate quick recoveries and selection processes maintain phenotypic and genetic stability throughout in-vitro aging of a culture. A CHO cell line also should be scalable to high-capacity culture…
Discover, Develop, Deliver
Astrea Bioseparations is the only adsorbent supplier that can discover new affinity ligands designed to bind selectively to a molecule of interest or specific impurity, develop efficient purification adsorbents and downstream methods, and deliver industrial-scale adsorbents (up to 1,000-L batch sizes) as loose slurry or in good manufacturing practice (GMP)-ready columns. With over 30 years of experience in development of affinity products and design and manufacture of new custom adsorbents, Astrea Bioseparations is a world leader in its field. The…
Retrofitting Your Bioreactor to Enhance Stirring Processes: Replacing Old Agitators with State-of-the-Art Magnetic Mixing Technology
A key challenge for companies involved in drug development is to meet the highest standards of sterile design and reliability. In this context, magnetic mixers offers many advantages for aseptic stirring processes compared to mechanically sealed agitators. ZETA not only supplies magnetic agitators for new bioreactors, but also supports its customers through the entire retrofitting process, from feasibility studies at the beginning to full process qualification and validation at the end. “Combat the risk of batch contamination in bioreactors and…
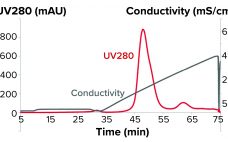
Reduce Downstream Processing Costs for MAbs By Switching to a Two-Step Platform
Downstream processing operations make up to 80% of the total costs for processing biotherapeutics. Given the current drive to reduce downstream costs, chromatographers and process engineers will need to streamline processes. Herein, we describe the benefits offered by using Tosoh’s two-step process for purifying monoclonal antibodies (MAbs) and compare that method with the standard industrial process. By combining high-performance protein A capture and a single polishing step on salt-tolerant anion-exchange resin, Tosoh’s approach can reduce downstream costs by 45% and…